ENA à¤à¤à¤¬à¤¾à¤à¤à¤¡ सà¥à¤à¥à¤°à¥à¤¨ à¤à¤²à¤¿à¤¸à¤¾ à¤à¤¿à¤
ENA à¤à¤à¤¬à¤¾à¤à¤à¤¡ सà¥à¤à¥à¤°à¥à¤¨ à¤à¤²à¤¿à¤¸à¤¾ à¤à¤¿à¤ Specification
- तापमान प्रतिरोध
- Store at 2–8°C
- विशेषताएँ
- Ready-to-use reagents, colorimetric detection, complete strips, quantitative and qualitative results
- सटीकता
- High sensitivity and specificity for ENA detection
- कंट्रोल टाइप
- Manual (standard ELISA protocol)
- शेप
- Rectangular kit box
- टाइप करें
- आयाम (एल* डब्ल्यू* एच)
- Standard kit size (Boxed), 96-well plate format
- उपकरण सामग्री
- High quality polystyrene microplate, reagent vials, buffer bottles
- मटेरियल
- Polystyrene, polypropylene (consumables), reagent grade chemicals
- एप्लीकेशन
- Laboratory diagnostics for autoimmune diseases, detection of ENA antibodies in serum or plasma
ENA à¤à¤à¤¬à¤¾à¤à¤à¤¡ सà¥à¤à¥à¤°à¥à¤¨ à¤à¤²à¤¿à¤¸à¤¾ à¤à¤¿à¤ Trade Information
- Minimum Order Quantity
- 5 Kits
- भुगतान की शर्तें
- कैश एडवांस (CA), कैश इन एडवांस (CID)
- आपूर्ति की क्षमता
- 50000 प्रति सप्ताह
- डिलीवरी का समय
- 7 दिन
- मुख्य निर्यात बाजार
- एशिया, ऑस्ट्रेलिया, मध्य अमेरिका, उत्तरी अमेरिका, दक्षिण अमेरिका, पूर्वी यूरोप, पश्चिमी यूरोप, मिडल ईस्ट, अफ्रीका
- मुख्य घरेलू बाज़ार
- ऑल इंडिया
About ENA à¤à¤à¤¬à¤¾à¤à¤à¤¡ सà¥à¤à¥à¤°à¥à¤¨ à¤à¤²à¤¿à¤¸à¤¾ à¤à¤¿à¤
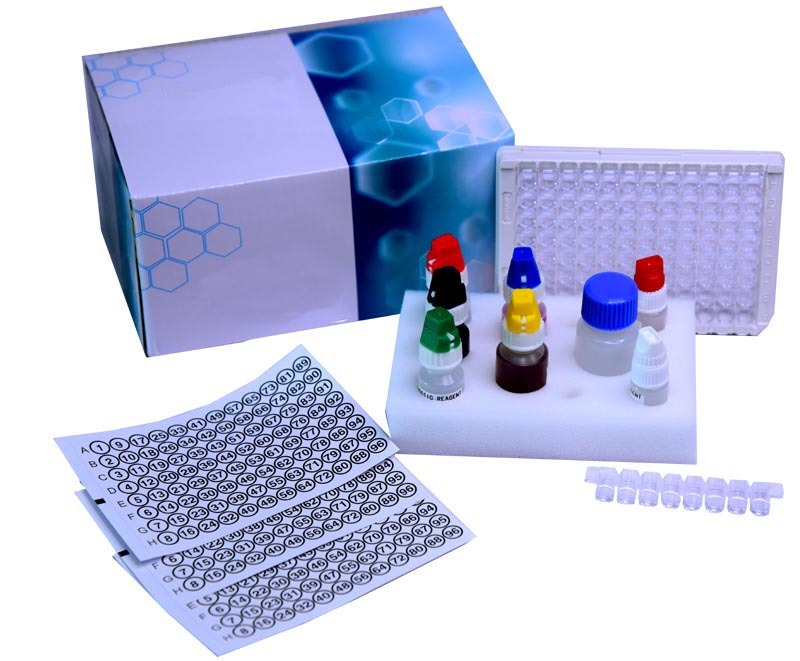
Unlock precision diagnostics with the ENA Combined Screen ELISA kita must-have for laboratories tackling autoimmune disorders. This dazzling, CE-marked kit delivers high sensitivity and specificity in detecting anti-ENA antibodies in human serum or plasma. Its trending, ready-to-use reagents and uncommon, imposing 96-well microplate format streamline the workflow, requiring just 90-120 minutes per assay. A full suite of controls is included, guaranteeing reliable results. Enjoy reagent stability at 28C, an impressive 1218 month shelf life, and quantifiable results for up to 96 tests, all in a compact, durable box. Manufactured and exported from India.
Superior Clinical Application for Autoimmune Diagnostics
The ENA Combined Screen ELISA kit is designed specifically for laboratory professionals seeking precise answers in autoimmune diagnostics. Its application surface allows for analysis of human serum or plasma, leveraging indirect ELISA methodology. Key features include ready-to-use reagents, impressive accuracy, and both qualitative and quantitative readouts. Its robust construction and complete strip format make it ideally suited for routine laboratory workflows, ensuring seamless integration and dependable results in autoimmune disease testing.
Domestic Market Reach, Rapid Dispatch, and Reliable Supply
With a strong presence in the Indian domestic market, the ENA Combined Screen ELISA kit is distributed through a network of trusted suppliers. Orders are processed and dispatched swiftly, often within standard shipment timelines. Supply ability is consistently maintained to meet demand rates from both large and modest healthcare facilities. This efficiency in delivery and shipment guarantees that crucial diagnostic resources are accessible when needed, supporting the ongoing needs of laboratories across India.
Superior Clinical Application for Autoimmune Diagnostics
The ENA Combined Screen ELISA kit is designed specifically for laboratory professionals seeking precise answers in autoimmune diagnostics. Its application surface allows for analysis of human serum or plasma, leveraging indirect ELISA methodology. Key features include ready-to-use reagents, impressive accuracy, and both qualitative and quantitative readouts. Its robust construction and complete strip format make it ideally suited for routine laboratory workflows, ensuring seamless integration and dependable results in autoimmune disease testing.
Domestic Market Reach, Rapid Dispatch, and Reliable Supply
With a strong presence in the Indian domestic market, the ENA Combined Screen ELISA kit is distributed through a network of trusted suppliers. Orders are processed and dispatched swiftly, often within standard shipment timelines. Supply ability is consistently maintained to meet demand rates from both large and modest healthcare facilities. This efficiency in delivery and shipment guarantees that crucial diagnostic resources are accessible when needed, supporting the ongoing needs of laboratories across India.
FAQs of ENA Combined Screen ELISA kit:
Q: How is the ENA Combined Screen ELISA kit used in laboratory diagnostics?
A: The kit is employed in clinical laboratories for the detection of anti-ENA antibodies in human serum or plasma, facilitating the diagnosis of various autoimmune diseases using an indirect enzyme-linked immunosorbent assay (ELISA) methodology.Q: What benefits does the ENA Combined Screen ELISA kit offer over traditional methods?
A: It provides high sensitivity and specificity, ready-to-use components, built-in positive and negative controls, and both quantitative and qualitative analysis, resulting in faster, more reliable autoimmune diagnostics.Q: When should the reagents in the ENA Combined Screen ELISA kit be used for best results?
A: For optimal performance, use the reagents before the expiration date and store them continuously at 28C in their original packaging. Assays should be performed within the stated 1218 month shelf life for greatest accuracy.Q: Where can this ELISA kit be applied?
A: The kit is primarily intended for laboratory use in clinical or research settings focusing on autoimmune disease diagnostics that require detection of anti-ENA antibodies in serum or plasma.Q: What is the process for analyzing samples with this kit?
A: Samples and controls are added to the 96-well plate, incubated with reagents, and processed according to the included manual, with absorbance read at 450 nm to determine antibody presence in about 90120 minutes.

Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email
अधिक Products in एलिसा किट Category
कार्डियोलिपिन आईजीजी एलिसा किट
माप की इकाई : किट/किट
न्यूनतम आदेश मात्रा : 10
मूल्य या मूल्य सीमा : आईएनआर
मूल्य की इकाई : किट/किट
उपयोग : Industrial
वारंटी : yes
लेप्टिन एलिसा किट
माप की इकाई : किट/किट
न्यूनतम आदेश मात्रा : 50
मूल्य या मूल्य सीमा : आईएनआर
मूल्य की इकाई : किट/किट
उपयोग : Industrial
वारंटी : yes
एएफपी एलिसा किट
माप की इकाई : किट/किट
न्यूनतम आदेश मात्रा : 10
मूल्य या मूल्य सीमा : आईएनआर
मूल्य की इकाई : किट/किट
उपयोग : Industrial
वारंटी : yes
17-ओएच प्रोजेस्टेरोन एलिसा किट
माप की इकाई : किट/किट
न्यूनतम आदेश मात्रा : 10
मूल्य या मूल्य सीमा : आईएनआर
मूल्य की इकाई : किट/किट





 जांच भेजें
जांच भेजें एसएमएस भेजें
एसएमएस भेजें
