TOXOPLASMA IgG/IgM RAPID TEST
TOXOPLASMA IgG/IgM RAPID TEST Trade Information
- Minimum Order Quantity
- 10 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 100000 Kits Per Week
- Delivery Time
- 7 Days
- Sample Available
- Yes
- Sample Policy
- If order is confirmed we will reimburse the sample cost
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
About TOXOPLASMA IgG/IgM RAPID TEST
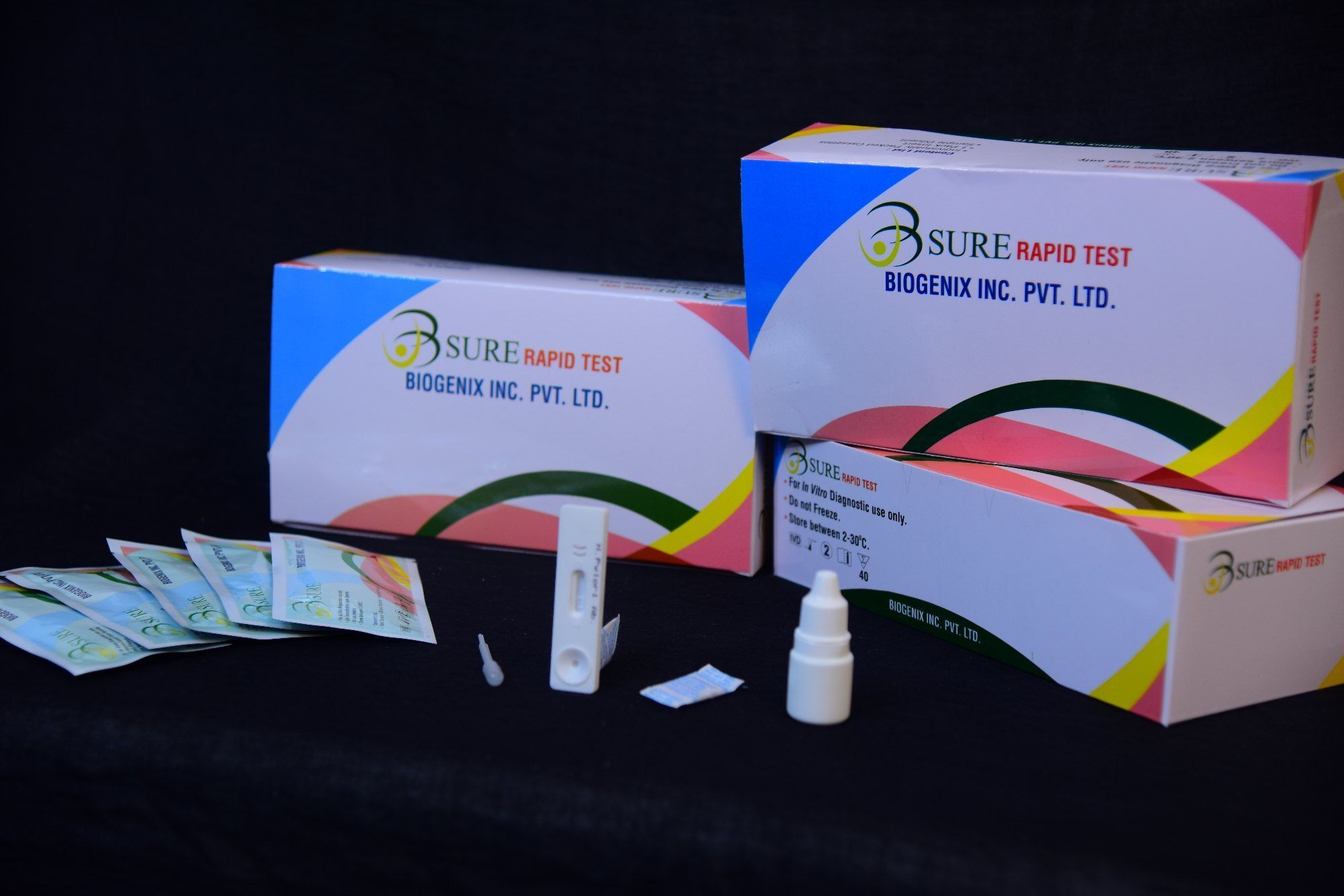
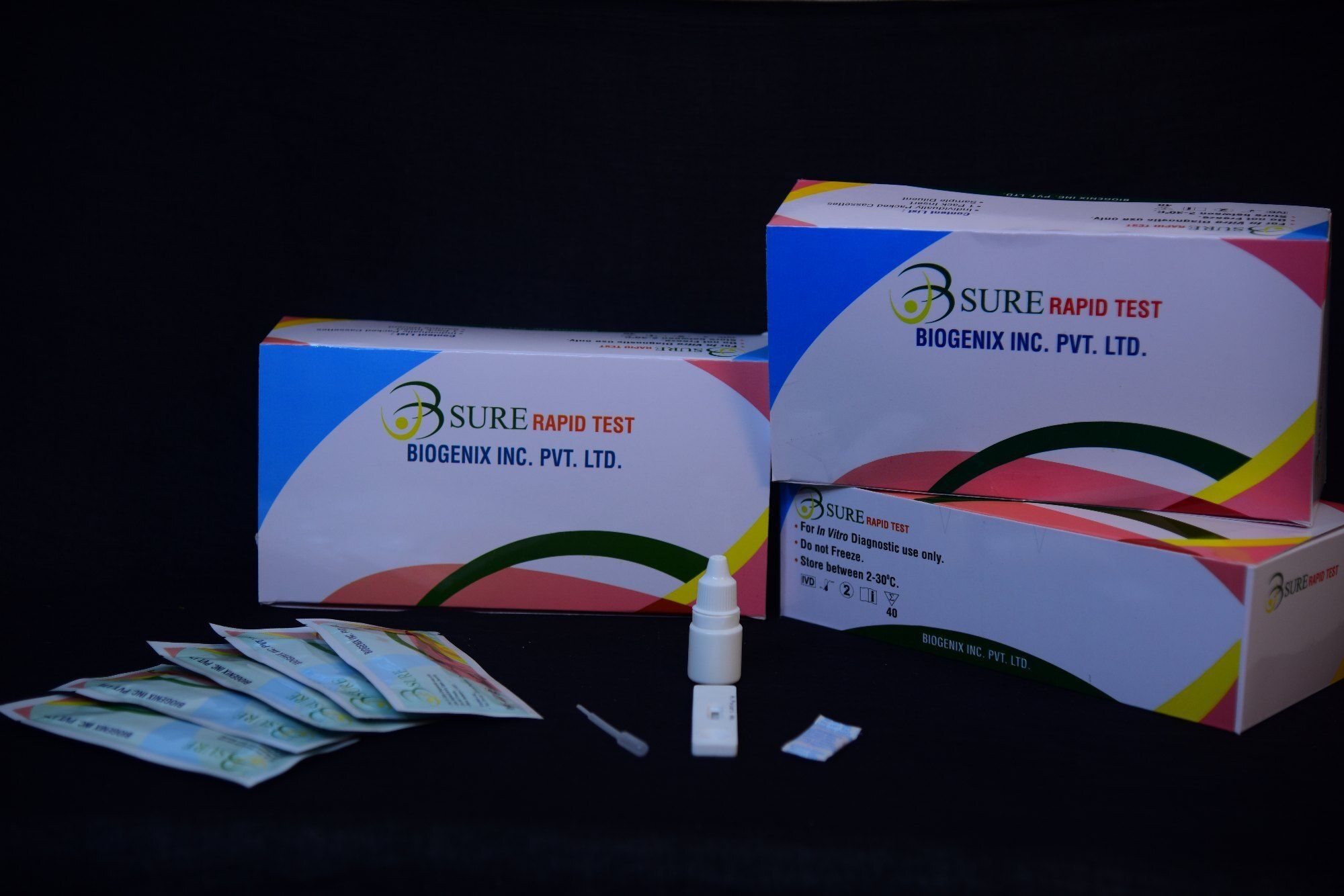
The Toxo IgG/IgM Combo Rapid Test is a lateral flow chromatographic immunoassay for the simultaneous detection and differentiation of IgG and IgM anti-Toxoplasma gondii (T. gondii) in human serum, plasma or whole blood.
Accurate and Quick Detection
The TOXOPLASMA IgG/IgM RAPID TEST provides reliable and fast results for the qualitative detection of Toxoplasma gondii antibodies. With a simple visual reading method and internal control included, this kit assures accuracy and helps facilitate early diagnosis. Its rapid turnaround time of 15 minutes makes it well-suited for busy diagnostic settings.
User-Friendly Design and Versatile Usage
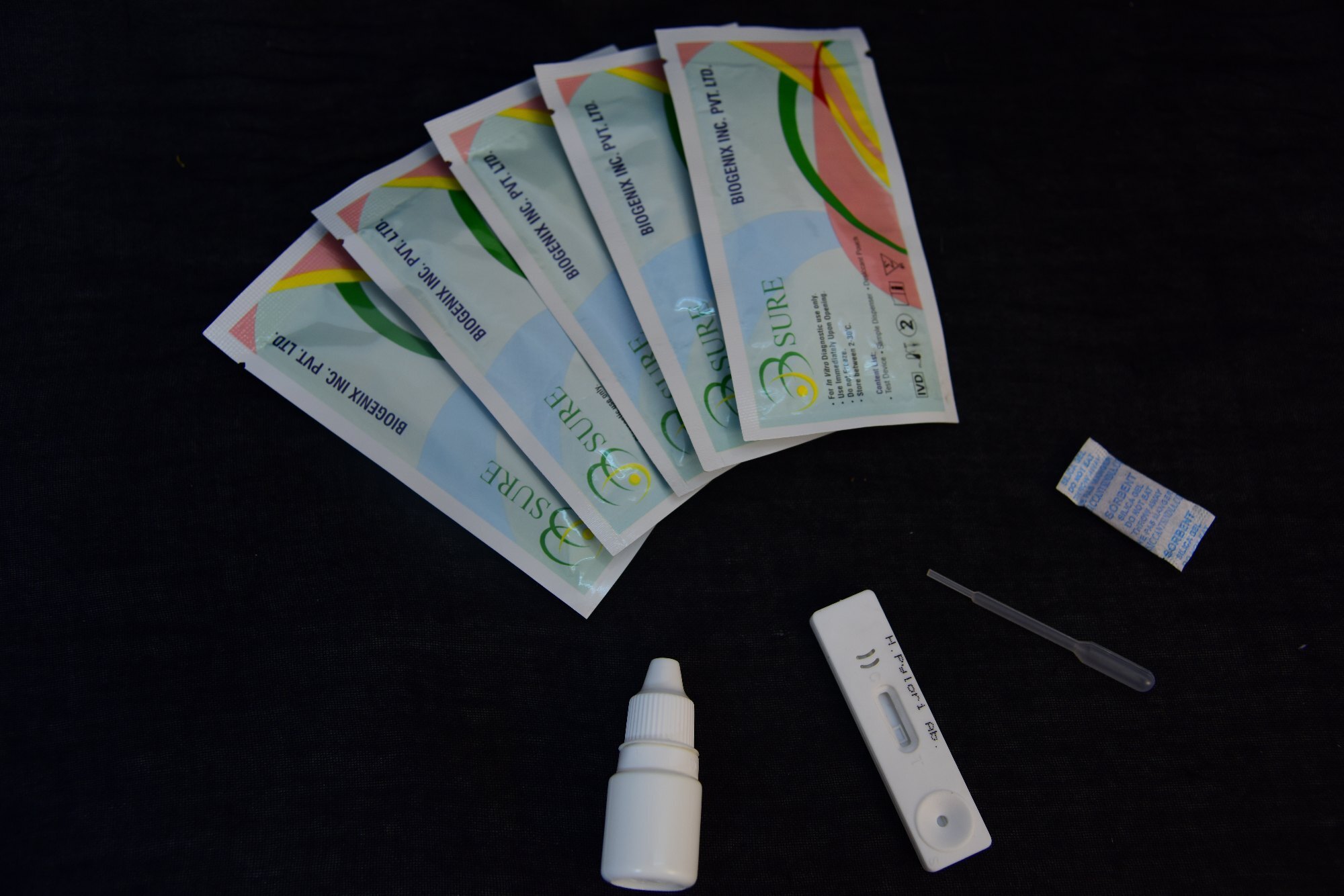
Packed in convenient cassettes, the kit accommodates serum, plasma, or whole blood samples, making it highly adaptable. Designed for straightforward use, each box includes clear instructions, sample pipettes, and buffer, allowing healthcare professionals to perform the test easily and efficiently.
Exceptional Quality and Compliance
This rapid test is CE marked and ISO 13485 certified, signifying adherence to global quality standards. Its storage flexibility (2-30C) and robust shelf life (1824 months) ensure product integrity, making it a reliable choice for medical facilities across India and for export.
FAQs of TOXOPLASMA IgG/IgM RAPID TEST:
Q: How does the TOXOPLASMA IgG/IgM RAPID TEST work?
A: The test employs a rapid immunochromatographic assay principle to qualitatively detect IgG and IgM antibodies against Toxoplasma gondii. Following sample addition to the cassette and buffer application, results are visually interpreted by the presence of distinct lines within 15 minutes.Q: What types of samples can be used with this test kit?
A: The test is compatible with serum, plasma, or whole blood samples, providing detection flexibility for different clinical settings.Q: When should the TOXOPLASMA IgG/IgM RAPID TEST be performed?
A: Testing should be performed when Toxoplasma gondii infection is suspected, such as during routine prenatal screening or in immunocompromised patients, for timely and efficient diagnosis.Q: Where should the test kits be stored for optimal performance?
A: Store the kits at a temperature between 2-30C, away from direct sunlight and moisture, to maintain their full shelf life and reliability.Q: What is the process for running the test and interpreting results?
A: Add the sample and buffer to the test cassette, wait 15 minutes, then visually inspect for the appearance of lines indicating IgG, IgM, and control. Presence of these lines corresponds to test results as explained in the provided instructions.Q: Is there any benefit to using this rapid test over laboratory-based methods?
A: Yes, the TOXOPLASMA IgG/IgM RAPID TEST offers quick results, high sensitivity and specificity (>99%), and can be conducted on-site without specialized equipment, allowing for faster clinical decision-making.Q: Are external controls included in the kit?
A: No, external controls are not supplied within the kit but are available separately if required for quality assurance protocols.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry Send SMS
Send SMS
