TROPONIN I RAPID TEST
TROPONIN I RAPID TEST Trade Information
- Minimum Order Quantity
- 10 Kits
- Payment Terms
- Paypal, Cash Advance (CA)
- Delivery Time
- 1 Week
- Sample Policy
- If order is confirmed we will reimburse the sample cost
- Main Domestic Market
- All India
About TROPONIN I RAPID TEST
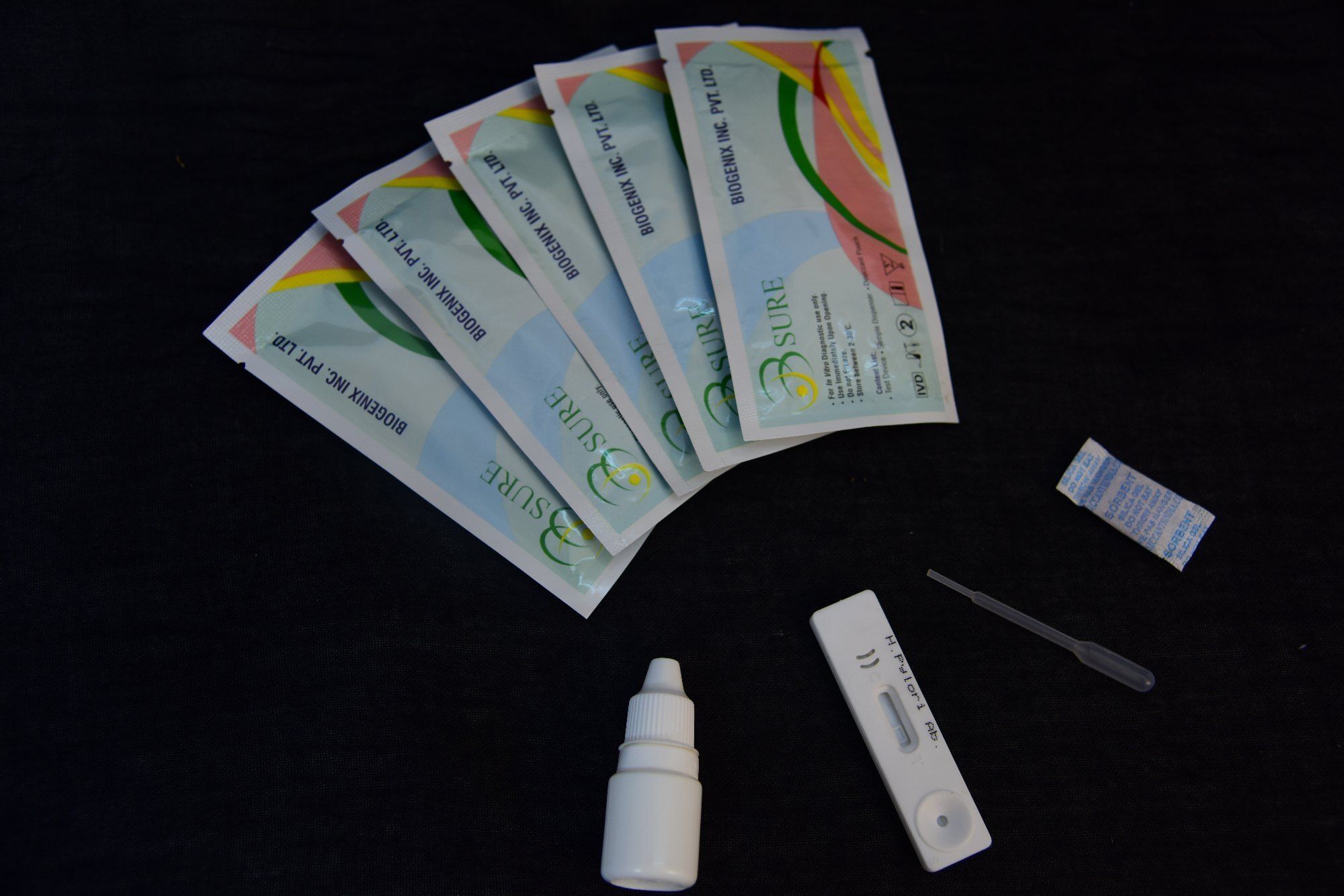
The Cardiac Troponin I Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of cardiac Troponin l(cTnl) in whole blood, serum or plasma. In this test procedure, capture reagent is immobilized in the test line region of the test. After specimen is added to the specimen area of the cassette, it reacts with anti-cTnl antibody coated colloid gold particles in the test. This mixture migrates chromato graphically along the length of the test and interacts with the immobilized capture reagent. The test format can detect cardiac Troponin l(cTnl) in specimens. If the specimen contains cardiac Troponin l(cTnl), a colored line will appear in the test line region, indicating a positive result. If the specimen does not contain cardiac Troponin (cTnl), a colored line will not appear in this region, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Rapid and Convenient Detection
This lateral flow immunoassay allows for quick screening of cardiac Troponin I with minimal sample volume. No instrumentation is required, making it an accessible tool for a wide range of healthcare environments, from emergency rooms to rural clinics.
Simple Visual Interpretation
Results are obtained visually, eliminating the need for specialized equipment. Each cassette yields clear, qualitative results in just 1015 minutes, empowering healthcare professionals to make prompt decisions regarding cardiac health.
Flexible Sampling and Robust Shelf Life
Compatible with whole blood, serum, or plasma samples, the test adapts to various clinical situations. With individually sealed pouches and a storage range from 2C to 30C, the product maintains integrity and reliability for up to 18 months.
FAQs of TROPONIN I RAPID TEST:
Q: How is the TROPONIN I RAPID TEST performed?
A: To perform the test, apply 10-15 L of whole blood, serum, or plasma to the sample well on the cassette. Add the supplied buffer, then wait 1015 minutes for the visual result to appear.Q: What does the TROPONIN I RAPID TEST detect?
A: The test qualitatively detects cardiac Troponin I, a biomarker elevated during myocardial injury, in whole blood, serum, or plasma specimens.Q: When should this test be used?
A: Use the TROPONIN I RAPID TEST when rapid detection of myocardial injury is clinically indicated, such as in chest pain emergencies or suspected acute coronary events.Q: Where can the TROPONIN I RAPID TEST be used?
A: This test is suitable for hospitals, emergency departments, clinics, and point-of-care settings, requiring no advanced laboratory equipment.Q: What is the process for reading the result?
A: After the sample and buffer are applied, observe the result window of the cassette. A visible line pattern will indicate either a positive or negative result, following the instructions supplied.Q: What are the main benefits of this rapid test?
A: The key benefits include quick turnaround (1015 minutes), ease of use, visual interpretation without instruments, and suitability for multiple sample types with a long shelf life.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+


 Send Inquiry
Send Inquiry Send SMS
Send SMS
