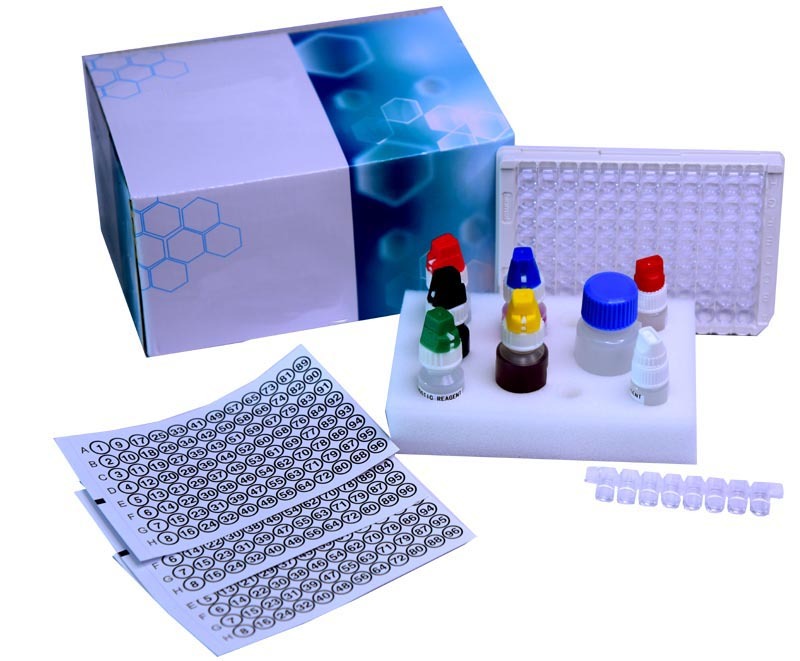
Epstein Barr Virus Early Antigen (EA) IgG ELISA kit
Epstein Barr Virus Early Antigen (EA) IgG ELISA kit Specification
- Accuracy
- High specificity and sensitivity
- Temperature Resistance
- Store at 2-8C
- Features
- Ready-to-use reagents, suitable for clinical laboratories
- Type
- ELISA Kit
- Equipment Type
- Immunoassay Kit
- Application
- Detection of Epstein-Barr Virus Early Antigen (EA) IgG antibodies
- Kit Components
- Coated microplate, Standards/controls, HRP conjugate, substrate, wash buffer, stop solution, instruction manual
- Shelf Life
- 12 months at recommended storage
- Lot Number
- Provided on kit
- Controls Included
- Positive and Negative controls
- Standard Curve
- Not required (qualitative)
- Specificity
- > 98%
- Sample Type
- Human serum or plasma
- Incubation Time
- 60-90 minutes total
- Compatible Equipment
- ELISA plate reader with 450 nm filter
- Assay Format
- 96-well microplate
- Regulatory Approvals
- For research use or diagnostic use depending on kit certification
- Sensitivity
- > 98%
- Detection Range
- Qualitative
- Test Principle
- Indirect enzyme-linked immunosorbent assay (ELISA)
Epstein Barr Virus Early Antigen (EA) IgG ELISA kit Trade Information
- Minimum Order Quantity
- 10 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Australia, Central America, Asia, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
About Epstein Barr Virus Early Antigen (EA) IgG ELISA kit
Specific Usage & Competitive Advantages
The Epstein Barr Virus Early Antigen (EA) IgG ELISA Kit is tailored for the qualitative detection of EA IgG antibodies, crucial in EBV research and diagnostics. With ready-to-use reagents, rapid 60-90 minute incubation, and high accuracy, it's ideal for clinical laboratories. Its indirect ELISA format saves time, eliminates the need for standard curves, and helps ensure reproducible results. Accurate controls and strong regulatory backing provide competitive advantages, meeting the high standards of the scientific community.
Sample Availability & Supply Policy
Premium samples of the Epstein Barr Virus Early Antigen (EA) IgG ELISA Kit are available at attractive rates, supporting evaluation and validation. Stock is ready to dispatch, ensuring swift delivery and reliable supply. Our sample policy enables researchers and laboratories to request and assess the product easily before bulk orders. Manufactured and exported in India, we promise robust supply ability to meet large-scale demands efficiently and consistently.
FAQ's of Epstein Barr Virus Early Antigen (EA) IgG ELISA kit:
Q: How does the Epstein Barr Virus EA IgG ELISA kit function?
A: The kit operates using an indirect enzyme-linked immunosorbent assay (ELISA) principle, enabling qualitative detection of Epstein Barr Virus Early Antigen IgG antibodies in human serum or plasma.Q: What types of samples are compatible with the EA IgG ELISA kit?
A: This kit is specifically designed for use with human serum or plasma, delivering high sensitivity and specificity in EBV antibody detection.Q: When should clinical laboratories use this ELISA kit?
A: It is best suited when rapid and accurate qualitative identification of EBV Early Antigen IgG antibodies is required in either research or diagnostic settings, depending on kit certification.Q: Where can I purchase or request samples of the EA IgG ELISA kit?
A: You can obtain this kit or request premium samples from authorized manufacturers, suppliers, or exporters in India, who ensure reliable stock and prompt supply.Q: What special features set this ELISA kit apart?
A: Key features include ready-to-use reagents, both positive and negative controls, a robust 96-well microplate format, and high specificity and sensitivity, making it superior for consistent lab results.Q: What is the process for running the EA IgG ELISA assay?
A: The process involves adding samples and controls to the coated microplate, incubating for 60-90 minutes, subsequent washing, adding HRP conjugate and substrate, stopping the reaction, and reading results at 450 nm with an ELISA plate reader.


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
Chikungunya IgM ELISA Kit
Price 27150 INR / Kit
Minimum Order Quantity : 10 Kits
Material : Plastic (microplate), glass (reagent bottles), aluminum pouch, paper inserts
Shape : Rectangular kit box
Accuracy : High sensitivity and specificity (98%)
Features : Readytouse reagents, colorcoded bottles, breakaway microplate strips, user manual
Mumps IgM ELISA kit
Price 21450 INR / Kit
Minimum Order Quantity : 50 Kits
Material : Kit includes plastic, bottles, foil pouches, disposable materials
Shape : Rectangular packaging box
Accuracy : High sensitivity and specificity as per kit insert
Features : Sensitive, Specific, Reliable, Readytouse reagents, Easytofollow protocol
Human IgG 90 Food ELISA Kit
Price 42500 INR / Kit
Minimum Order Quantity : 5 Kits
Material : Reagent grade chemicals, polystyrene plate
Shape : Rectangular kit box
Accuracy : Highly sensitive (Detection limit <10 ng/mL)
Features : Readytouse, easy protocol, validated for multifood matrices
Thyroglobulin ELISA Kit
Price 13500 INR / Kit
Minimum Order Quantity : 1 Kit
Material : Highgrade diagnostic plastics
Shape : Rectangular packaging
Accuracy : High sensitivity and specificity
Features : Readytouse reagents, colorimetric detection, easy protocol, validated controls





 Send Inquiry
Send Inquiry Send SMS
Send SMS
