WIDAL TEST
WIDAL TEST Specification
- Product Name
- WIDAL TEST
- Components
- Antigen suspensions (O, H, AH, BH)
- Type
- Slide Agglutination Test
- Intended Use
- Serodiagnosis of enteric fever
- Quality Assurance
- Batch tested and validated
- Storage Temperature
- 2°C - 8°C
- Usage
- Clinical diagnostic for detecting Salmonella antibodies
- Test Principle
- Agglutination
- Hazardous
- No
- Packaging Size
- 1 x 5 ml vial
- Reading Method
- Visual observation of agglutination
- Sample Type
- Serum
- Shelf Life
- 12 months
About WIDAL TEST
Widal test. Purpose. serologicaltestfor enteric fever. In 1896 and named after its inventor, Georges-FernandWidal, is a presumptive serologicaltestfor enteric fever or undulant fever whereby bacteria causing typhoid fever is mixed with a serum containing specific antibodies obtained from an infected individual.Reliable Serodiagnosis for Enteric Fever
The WIDAL TEST offers an effective and straightforward approach for detecting antibodies to Salmonella, aiding in the diagnosis of enteric fever. Its slide agglutination format allows clinical laboratories to quickly and visually identify positive samples, which is essential for timely treatment decisions.
Comprehensive and Easy-to-Use Kit
Each kit includes ready-to-use antigen suspensions (O, H, AH, BH) and ensures easy handling and preparation. The visual agglutination method eliminates the need for specialized instrumentation, making the process convenient for clinicians and laboratory technicians.
Quality Assurance and Shelf Life
Every batch of the WIDAL TEST is thoroughly validated and tested for performance assurance. The reagents maintain integrity for up to 12 months when stored at 2C - 8C, allowing healthcare providers to rely on consistent and accurate results throughout its shelf life.
FAQs of WIDAL TEST:
Q: How is the WIDAL TEST performed in the laboratory?
A: The WIDAL TEST is conducted by mixing a patients serum sample with specific antigen suspensions (O, H, AH, BH) on a slide and observing for agglutination. The presence of visible clumping indicates antibodies against Salmonella, confirming a positive result.Q: What type of sample is required for the WIDAL TEST?
A: This test requires a serum sample, which should be collected from the patient using standard aseptic methods prior to the test.Q: When should the WIDAL TEST be used in clinical practice?
A: The test is most effective when enteric fever (typhoid or paratyphoid) is suspected based on clinical symptoms and a history of exposure, providing valuable diagnostic support in the acute phase of illness.Q: Where can the WIDAL TEST be stored and for how long?
A: The WIDAL TEST reagents must be stored at a temperature range of 2C to 8C, and they retain their validity for 12 months from the date of manufacture when stored correctly.Q: What is the process to interpret the results of the WIDAL TEST?
A: After adding the serum to the antigen suspensions and mixing, results are read by visually inspecting for agglutination. Distinct clumping within a few minutes indicates a positive reaction, suggesting the presence of Salmonella antibodies.Q: How does the WIDAL TEST benefit clinical diagnostics?
A: The test provides a rapid, reliable, and easy-to-interpret method for detecting enteric fever, allowing for timely diagnosis and management, and reducing the need for more complex laboratory infrastructure.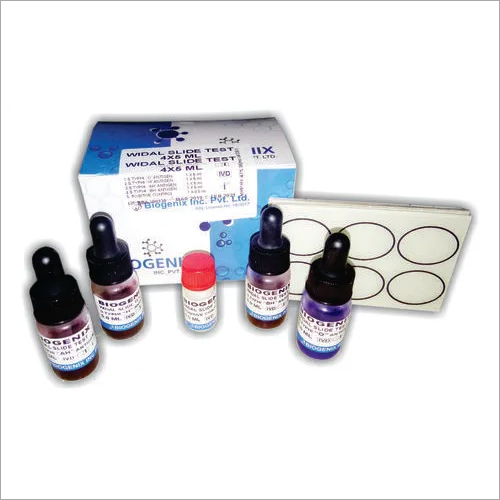
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email




 Send Inquiry
Send Inquiry Send SMS
Send SMS
