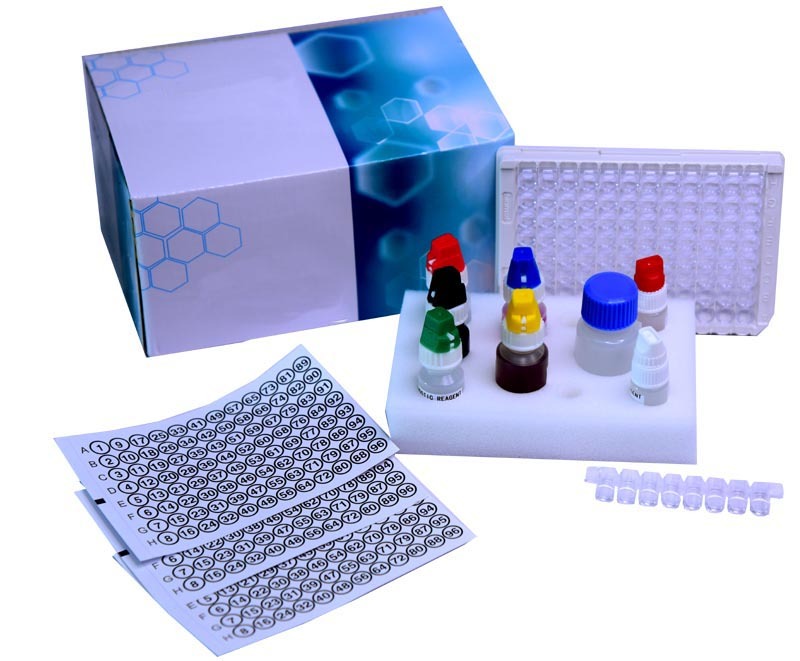
Gliadin IgA ELISA kit
Gliadin IgA ELISA kit Specification
- Accuracy
- High Sensitivity and Specificity
- Temperature Resistance
- 2-8C (Storage)
- Features
- Ready-to-use reagents, high precision, rapid results, clear protocol
- Control Type
- Manual (Microplate based)
- Shape
- Kit packaging
- Type
- ELISA Kit
- Dimension (L*W*H)
- Standard kit box
- Equipment Type
- Diagnostics ELISA kit
- Equipment Materials
- Reagent grade plastics and bottles
- Material
- Biological reagents, microplate
- Application
- Detection of Gliadin IgA antibodies in human serum and plasma
- Incubation Time
- 1.52 hours
- Detection Range
- Qualitative/Quantitative (as per kit instructions)
- Pack Size
- 1 x 96 tests
- Test Principle
- Antigen-antibody reaction, colorimetric detection
- Intended User
- For In Vitro Diagnostic Use (IVD) only
- Controls Included
- Positive and Negative Controls
- Shelf Life
- 12 months at 2-8C
- Detection Method
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Sample Type
- Human Serum/Plasma
- Assay Format
- 96-well microplate
Gliadin IgA ELISA kit Trade Information
- Minimum Order Quantity
- 5 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
About Gliadin IgA ELISA kit
Experience an exclusive Price Cut on our redoubtable Gliadin IgA ELISA Kit! Special Clearance available for manufacturers, exporters, and suppliers across India. With a heroic detection method-Enzyme-Linked Immunosorbent Assay (ELISA)-this kit exhibits terrific accuracy for qualitative and quantitative measurement of Gliadin IgA antibodies in human serum/plasma. Featuring a convenient 96-well microplate format, rapid incubation (1.5-2 hours), and ready-to-use reagents, the kit provides high sensitivity, specificity, and a clear protocol. Controls included ensure reliability, while reagent-grade materials withstand 2-8C storage for up to 12 months. Ideal for in vitro diagnostic use.
Site Application and Competitive Advantage
The Gliadin IgA ELISA kit is primarily designed for use in diagnostic laboratories specializing in autoimmune or celiac disease testing. Its specific application focuses on detecting IgA antibodies against gliadin in human serum or plasma samples. Laboratories benefit from the kit's high precision, rapid results, manual microplate-based control, and clear protocol-making it an outstanding choice amidst competitors. It stands out for ready-to-use reagents and robust shelf life, ensuring consistent, reliable outcomes for diagnostic professionals.
Certifications, Sample Availability, and Domestic Market
We proudly offer the Gliadin IgA ELISA Kit certified for in vitro diagnostic applications, adhering to global standards. Samples are available upon request to facilitate evaluation before purchase, with asking price details provided through our responsive order processing system. Serving all major regions domestically, we provide efficient and prompt delivery to laboratories and hospitals nationwide. Take advantage of our exclusive offer and enhance your diagnostic accuracy with a trusted, certified solution.
Site Application and Competitive Advantage
The Gliadin IgA ELISA kit is primarily designed for use in diagnostic laboratories specializing in autoimmune or celiac disease testing. Its specific application focuses on detecting IgA antibodies against gliadin in human serum or plasma samples. Laboratories benefit from the kit's high precision, rapid results, manual microplate-based control, and clear protocol-making it an outstanding choice amidst competitors. It stands out for ready-to-use reagents and robust shelf life, ensuring consistent, reliable outcomes for diagnostic professionals.
Certifications, Sample Availability, and Domestic Market
We proudly offer the Gliadin IgA ELISA Kit certified for in vitro diagnostic applications, adhering to global standards. Samples are available upon request to facilitate evaluation before purchase, with asking price details provided through our responsive order processing system. Serving all major regions domestically, we provide efficient and prompt delivery to laboratories and hospitals nationwide. Take advantage of our exclusive offer and enhance your diagnostic accuracy with a trusted, certified solution.
FAQ's of Gliadin IgA ELISA kit:
Q: How does the Gliadin IgA ELISA kit detect antibodies in serum or plasma samples?
A: The kit utilizes an antigen-antibody reaction followed by colorimetric detection within a 96-well microplate format. This process identifies and quantifies IgA antibodies against gliadin in human serum or plasma samples, following a clear, manual protocol.Q: What makes the Gliadin IgA ELISA kit a competitive option for diagnostic laboratories?
A: Its high sensitivity, specificity, rapid incubation time (1.5-2 hours), and ready-to-use reagents deliver reliable and efficient results. The inclusion of both positive and negative controls further enhances accuracy and confidence in test outcomes.Q: When is the best time to order a sample of the Gliadin IgA ELISA kit?
A: You can request a sample at any time before placing your main order. Our order processing team will promptly handle your request and provide all relevant pricing and product details.Q: Where can the Gliadin IgA ELISA kit be applied?
A: The kit is intended for use in diagnostic laboratories, hospitals, or medical research facilities focusing on autoimmune disease or celiac disease screening in human patients.Q: What are the benefits of using a manual, microplate-based ELISA kit?
A: Manual microplate-based kits enable greater user control, precision, and flexibility in assay execution, ensuring consistently high-quality results and adaptability to laboratory workflow.

Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in ELISA Kits Category
5- HIAA Elisa Kit
Price 72750 INR / Kit
Minimum Order Quantity : 50 Kits
Material : Biological reagents, Polystyrene microplate
Shape : Rectangular kit box
Thyroglobulin ELISA Kit
Price 13500 INR / Kit
Minimum Order Quantity : 1 Kit
Material : Highgrade diagnostic plastics
Shape : Rectangular packaging
Varicella-Zoster IgM ELISA Test
Price 20550.00 INR / Kit
Minimum Order Quantity : 50 Kits
Material : Plastic
Shape : Rectangular
Warranty : yes
Color : multi color
Adenovirus IgA ELISA kit
Price 64500.00 INR / Kit
Minimum Order Quantity : 5 Kits
Material : Plastic
Shape : Rectangular
Warranty : yes
Color : multi color





 Send Inquiry
Send Inquiry Send SMS
Send SMS
