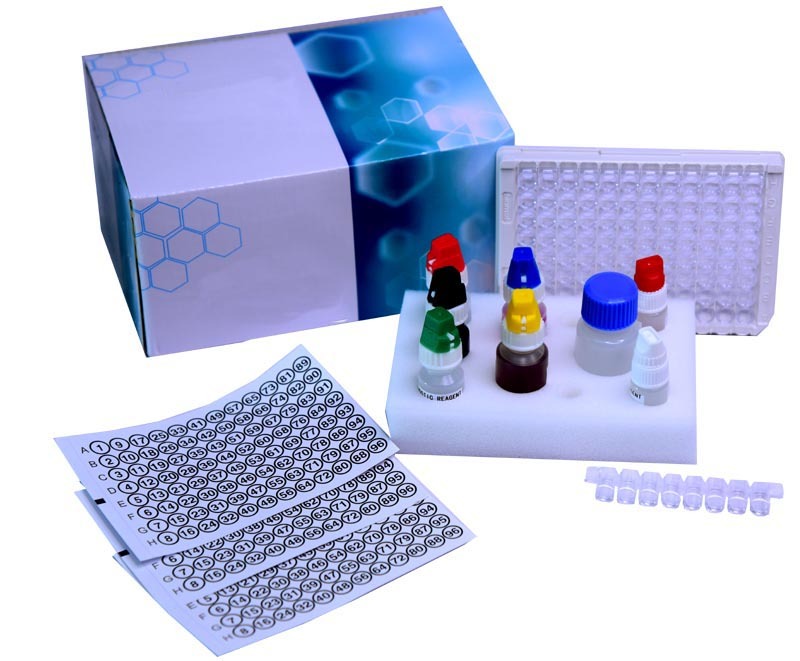
West Nile IgM ELISA kit
West Nile IgM ELISA kit Specification
- Temperature Resistance
- Store at 2-8C, do not freeze
- Shape
- Rectangular kit box
- Accuracy
- High sensitivity and specificity for IgM antibodies
- Control Type
- Manual processing with incubation steps
- Features
- Ready-to-use reagents, colorimetric detection, includes positive and negative controls, easy-to-follow protocol
- Type
- ELISA Kit
- Dimension (L*W*H)
- Standard kit box size (approx. 24cm x 18cm x 7cm)
- Equipment Type
- Immunoassay diagnostic kit
- Equipment Materials
- High quality plastics and reagents, pre-coated 96-well microplate
- Material
- Biological reagents, microtiter plate, buffers, conjugate, substrate
- Application
- Qualitative detection of IgM antibodies to West Nile Virus in human serum or plasma
West Nile IgM ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Eastern Europe, Western Europe, Middle East, Africa, Asia, Australia, Central America, North America, South America
About West Nile IgM ELISA kit
Application Areas and Methodology
The West Nile IgM ELISA kit is ideally suited for clinical laboratories, research facilities, and diagnostic centers. Its application encompasses both targeted screening and broader surveillance studies for West Nile Virus exposure. With a straightforward indirect ELISA format, the kit employs colorimetric detection (450 nm) using human plasma or serum samples. The easy-to-follow manual incubation process makes it valuable for rapid, qualitative IgM antibody detection, supporting both specialized and general use cases in immunodiagnostic workflows.
Domestic Market Features and Delivery Information
Our West Nile IgM ELISA kit is readily available for sale within major domestic markets across India. Packing & Dispatch operations are streamlined, ensuring safe and prompt delivery-typically within 5-7 business days of order confirmation. Clients may request a quotation for current sale price and special sample policies. Each kit is packed securely in a standard, rectangular box to preserve integrity during transit and storage, guaranteeing all materials arrive in optimum condition for immediate use.
FAQ's of West Nile IgM ELISA kit:
Q: How does the West Nile IgM ELISA kit detect antibodies in human samples?
A: The kit utilizes the indirect ELISA technique, where human serum or plasma is added to a pre-coated microplate. After a series of incubation and washing steps, a colorimetric reaction occurs, allowing the detection of West Nile IgM antibodies at 450 nm.Q: What are the specific uses and benefits of this ELISA kit?
A: The ELISA kit specializes in the qualitative detection of IgM antibodies to West Nile Virus, helping in both clinical diagnostics and epidemiological research. It offers high sensitivity and specificity, ready-to-use reagents, and a straightforward protocol with reliability ensured by strict quality control.Q: When can I expect delivery and what is the usual packaging?
A: Orders are typically dispatched within 5-7 business days. Each kit is securely packed in a standard rectangular box (approx. 24 cm x 18 cm x 7 cm) to protect contents during transit and storage.Q: Where is this kit primarily available and who is the manufacturer?
A: The West Nile IgM ELISA kit is exported, manufactured, and supplied primarily within India, serving both domestic and select international clients. Inquiries for supply outside India should be directed to the manufacturer or designated exporter.Q: What control types and equipment are needed for the usage process?
A: The kit requires manual control processing with defined incubation steps. Essential equipment includes a microplate reader for colorimetric measurement and micropipettes for sample handling, providing accurate and reproducible detection results.Q: How is kit quality assured before sale and what is the storage recommendation?
A: Each kit undergoes comprehensive quality control testing before dispatch to ensure accuracy and reliability. For optimal preservation, the kit should be stored at 2-8C and must not be frozen.


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
25 OH Vitamin D ELISA Kit
Price 28500 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Rectangular Microplate
Material : HighGrade Reagents and Microplate
Equipment Type : ELISA Kit
Application : Quantitative Determination of 25OH Vitamin D in Human Serum or Plasma
Calcitonin ELISA Kit
Price 46500 INR / Kit
Minimum Order Quantity : 5 Kits
Shape : Rectangular microplate
Material : Highgrade laboratory plastics and chemicals
Equipment Type : Immunoassay kit
Application : Quantitative detection of Calcitonin in biological samples
Dihydro-Testo sterone ELISA Kit
Price 47550 INR / Kit
Minimum Order Quantity : 5 Kits
Shape : Rectangular Microplate
Material : Plastic wells, reagent solutions
Equipment Type : ELISA Microplate Kit
Application : Quantitative detection of DihydroTestosterone (DHT) in serum, plasma, and other biological samples
Normetanephrine Urine ELISA Kit (Fast Track)
Price 72750.00 INR / Kit
Minimum Order Quantity : 1 Kit
Shape : Rectangular
Material : Plastic





 Send Inquiry
Send Inquiry Send SMS
Send SMS
