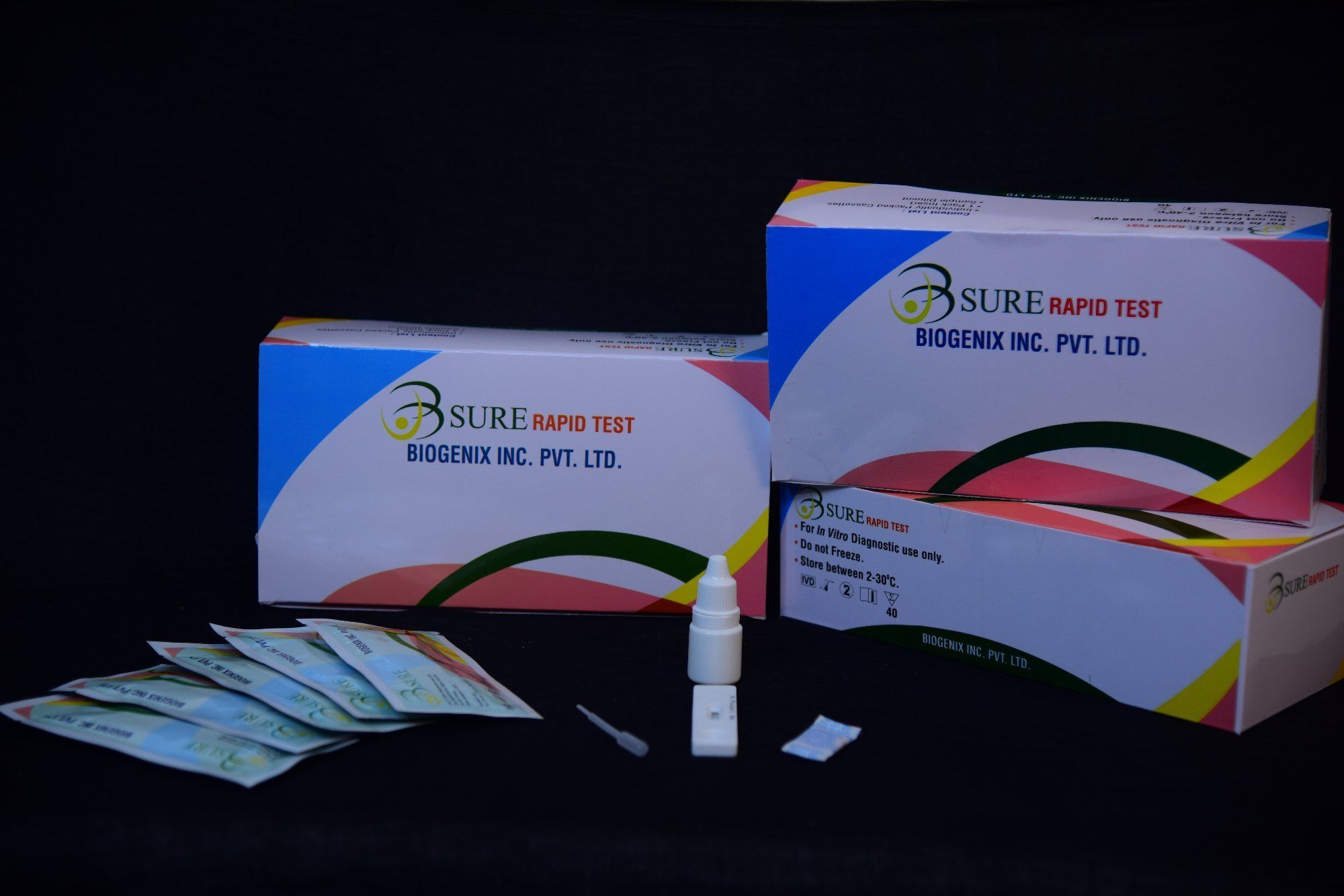
HAV IgM RAPID TEST
HAV IgM RAPID TEST Specification
- Specimen
- Serum / Plasma / Whole Blood
- Packaging
- Box of 25 tests
- Result Time
- 15-20 minutes
- Format
- Rapid Test / Cassette
- Specificity
- >99%
- Kit Includes
- Test cassettes, dropper, buffer, package insert
- Transport Condition
- Room temperature
- Quality Control
- Built-in procedural control line
- Intended Use
- For in vitro diagnostic use only
- Sample Volume Required
- 10L (serum/plasma) or 20L (whole blood)
- Sensitivity
- >99%
- Detection
- Hepatitis A IgM Antibody
- User Level
- Trained healthcare professionals
- Shelf Life
- 18-24 months
- Product Name
- HAV IgM RAPID TEST
- Reading Method
- Visual interpretation
- Storage Condition
- 230C
HAV IgM RAPID TEST Trade Information
- Minimum Order Quantity
- 10 Kits
- Supply Ability
- 100000 Kits Per Week
- Delivery Time
- 10 Days
- Sample Policy
- If order is confirmed we will reimburse the sample cost
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
About HAV IgM RAPID TEST
The HAV IgM Cassette Rapid Test is a lateral chromatographic immunoassay.
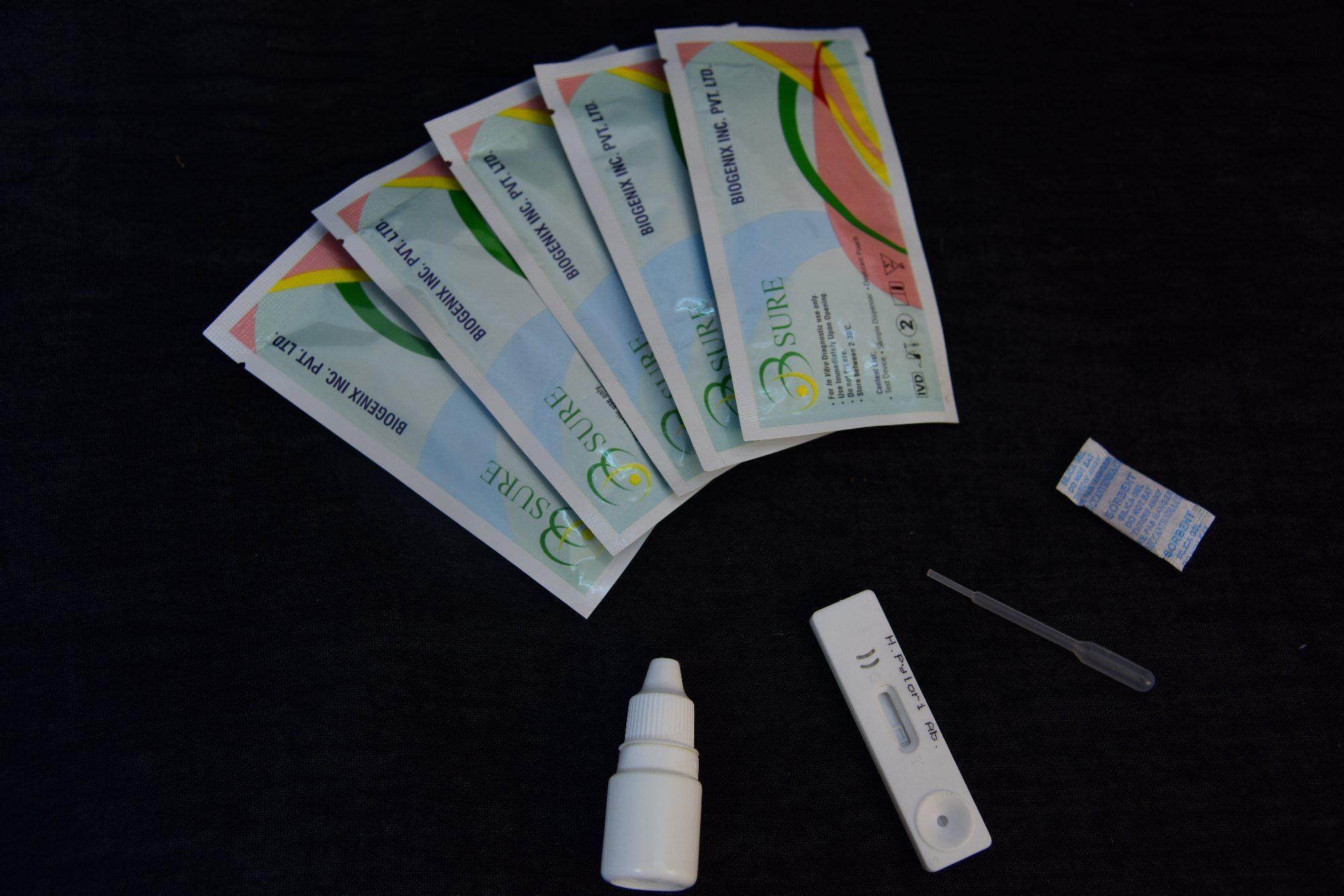
The test cassette consists of: 1) a burgundy colored conjugate pad containing mouse anti- human IgM antibody conjugated with colloidal gold (IgM conjugates) and 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The T band is pre-coated with recombinant HAV antigen, and the C band is pre-coated with goat anti-mouse IgM antibodies. When an adequate volume of test specimen is dispensed into the sample well of the cassette, the specimen migrates by capillary action across the cassette. Anti -HAV IgM if present in the specimen will bind to the IgM conjugates. The immuno complex is then captured on the membrane by the pre-coated HAV antigen forming a burgundy colored T line, indicating a HAV IgM positive test result. Absence of the T line suggests a negative result. The test contains an internal control (C line) which should exhibit a burgundy colored line of the immunocomplex of goat anti-mouse IgG/IgM-gold conjugate regardless of the color development of the T line. Otherwise, the test result is invalid and the specimen must be retested with another device.
Accurate Hepatitis A Diagnosis Made Easy
The HAV IgM Rapid Test offers healthcare professionals a streamlined solution for detecting acute Hepatitis A infections. Its high sensitivity and specificity ensure dependable results, while the simple drop-and-read format supports efficient workflow in medical laboratories. Designed for use with multiple specimen typesserum, plasma, or whole bloodthe test is convenient for varied clinical scenarios. An integrated control line verifies proper function with every use.
Simple Testing Procedure for Quick Turnaround
Using the HAV IgM Rapid Test involves collecting a small specimen (10L for serum/plasma or 20L for whole blood), applying it onto the cassette with the provided dropper, adding buffer, and waiting 1520 minutes for visual interpretation. The inclusion of a control line guarantees procedural accuracy, and results can be easily interpreted on-site without specialized equipment.
FAQs of HAV IgM RAPID TEST:
Q: How is the HAV IgM Rapid Test used for detecting Hepatitis A infection?
A: The HAV IgM Rapid Test is used by applying a serum, plasma, or whole blood sample onto the cassette, followed by buffer addition. After 15-20 minutes, healthcare professionals visually interpret the results for Hepatitis A IgM antibodies.Q: What benefits does the HAV IgM Rapid Test offer over traditional laboratory methods?
A: This rapid test provides results in 1520 minutes, requires minimal sample volume, and needs no advanced equipment. Its outstanding sensitivity and specificity facilitate early Hepatitis A diagnosis, enabling prompt intervention and care.Q: When can the test be performed after suspected exposure to Hepatitis A?
A: The HAV IgM Rapid Test can be performed when a patient presents symptoms suggestive of acute Hepatitis A or has known exposure. Consult a healthcare professional for optimal timing based on clinical presentation.Q: Where can the HAV IgM Rapid Test be stored and transported safely?
A: The test kits should be stored at 230C and can be transported at room temperature. Proper handling maintains test integrity throughout its 1824 month shelf life.Q: What is included in each HAV IgM Rapid Test kit?
A: Each kit contains 25 test cassettes, droppers, buffer solution, and a package insert detailing usage instructions and interpretation guidelines.Q: Who should perform the HAV IgM Rapid Test, and is special training required?
A: This test is intended for trained healthcare professionals familiar with specimen collection and diagnostic procedures to ensure accurate results and patient safety.Q: How does the built-in quality control line help users during testing?
A: The control line appears for every valid test, confirming correct test execution and indicating that reagents are functioning properly. This feature supports reliable interpretation and procedural compliance.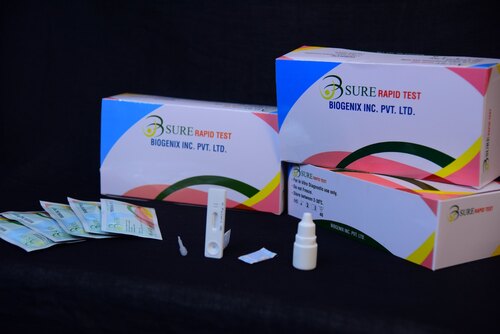

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Rapid Test Kit Category
EDAN Calibrant Fluid - Arterial Blood Gas (ABG) Analyzer
Price 8500 INR / Pack
Minimum Order Quantity : 1 Pack Pack


 Send Inquiry
Send Inquiry Send SMS
Send SMS
