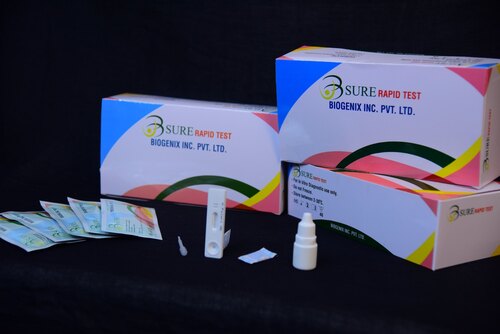
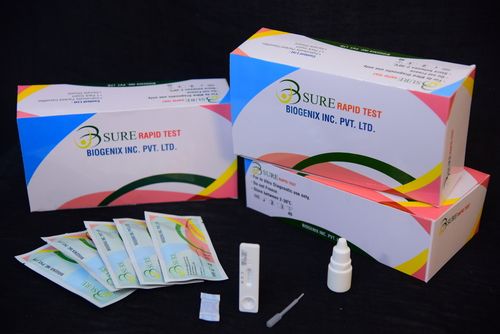
HBsAg Rapid Test
HBsAg Rapid Test Trade Information
- Minimum Order Quantity
- 200 Kits
- Payment Terms
- Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 100000 Kits Per Month
- Delivery Time
- 7 Days
- Sample Available
- Yes
- Sample Policy
- If order is confirmed we will reimburse the sample cost
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
About HBsAg Rapid Test
BIPL HBsAg Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of hepatitis B surface antigen (HBsAg) in human serum/plasma at a level equal to or higher than 0.5 ng/mL. It is intended to be used as a screening test and as an aid in the diagnosis of infection with hepatitis B virus (HBV). Any reactive specimen with the BIPL HBsAg Rapid Test must be confirmed with alternative testing method(s) and clinical findings.
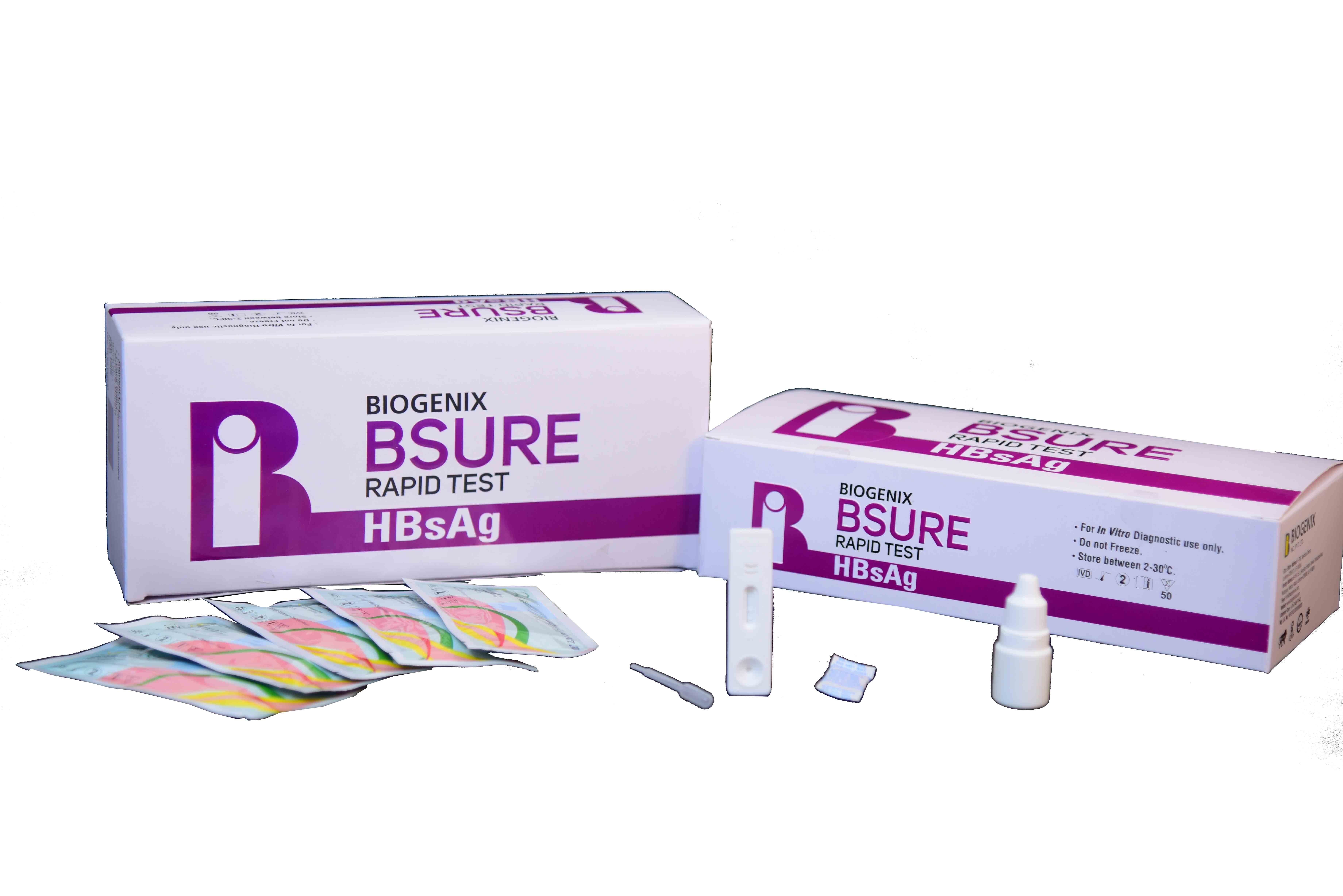
BIPL HBsAg Rapid Test is a lateral flow chromatographic immunoassay. The test cassette consists of:
1) a burgundy colored conjugate pad containing mouse anti-HBsAg antibody conjugated with colloidal gold (HBsAg Ab conjugates) and a control antibody conjugated with colloidal gold,
and
2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre-coated with non-conjugated HBsAg antibody, and the C line is pre-coated with a control line antibody.
Precise and Rapid HBV Screening
Utilizing advanced immunochromatographic technology, the HBsAg Rapid Test provides quick and reliable detection of hepatitis B surface antigen, facilitating timely clinical decision-making. The easy-to-use kit enables medical professionals to obtain accurate results in as little as 15 minutes.
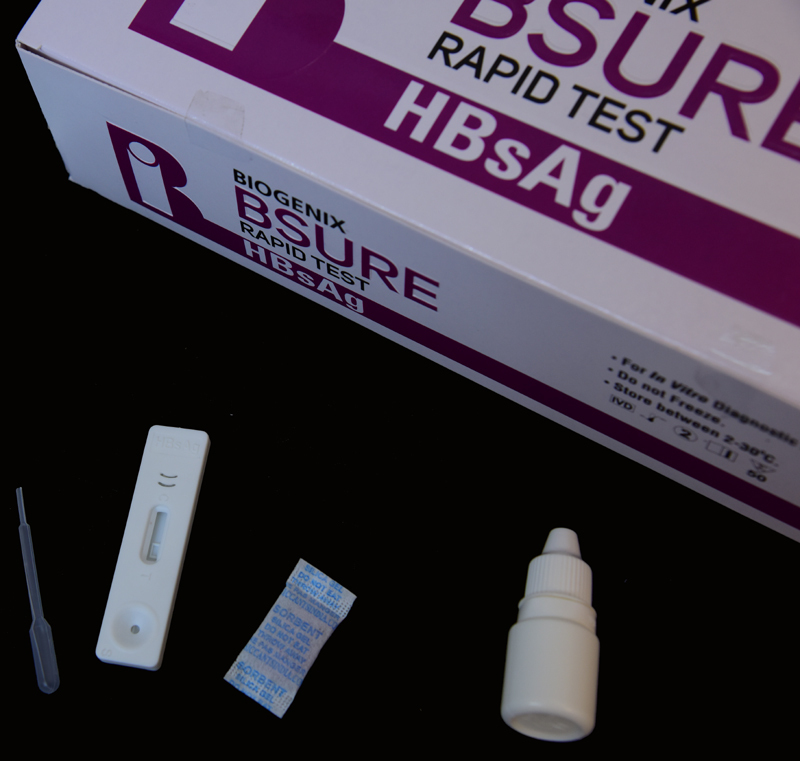
Complete Kit for Professional Use
This diagnostic test set is supplied as a box containing all necessary componentstest device, buffer, dropper, and a clear instruction manual. Designed for use by trained professionals, the kit ensures convenient and error-free operation in laboratory and healthcare environments.
FAQs of HBsAg Rapid Test:
Q: How is the HBsAg Rapid Test performed using the provided kit?
A: To conduct the test, add 23 drops (60100 L) of serum, plasma, or whole blood onto the test device, followed by the buffer. Refer to the instruction manual for detailed procedure. Read results visually within 1520 minutes.Q: What is the benefit of using an immunochromatographic assay for HBV surface antigen detection?
A: The immunochromatographic assay offers high sensitivity (99.0%) and specificity (99.5%), ensuring reliable qualitative results. It provides rapid screening, allowing healthcare professionals to quickly identify HBV infections.Q: When should the result from the HBsAg Rapid Test be interpreted?
A: Results should be read visually within 1520 minutes after sample application for accurate interpretation. Reading results outside of this time frame may lead to incorrect conclusions.Q: Where is this HBsAg Rapid Test intended to be used?
A: This test is designed for professional in-vitro diagnostic use, suitable for hospital laboratories, clinics, or diagnostic centers. It is not intended for home or non-professional use.Q: What specimens can be used with the HBsAg Rapid Test, and what sample volume is required?
A: The test is validated for serum, plasma, or whole blood samples. A volume of 2-3 drops (approximately 60100 L) is required for each test.Q: How should the HBsAg Rapid Test kit be stored to ensure reliability during its 24-month shelf life?
A: Store the kit at temperatures between 230C, away from direct sunlight and moisture. Proper storage ensures the kit maintains its validity throughout the 24-month shelf life.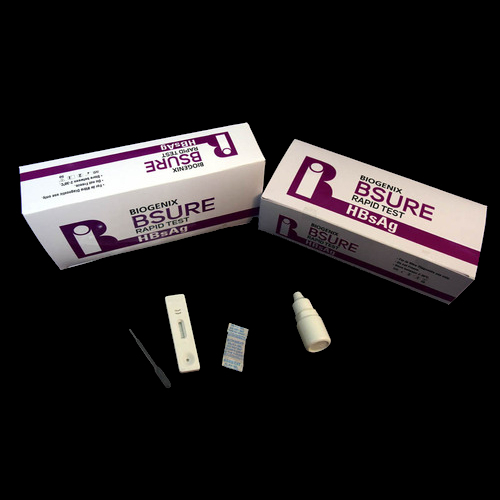

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



 Send Inquiry
Send Inquiry Send SMS
Send SMS
