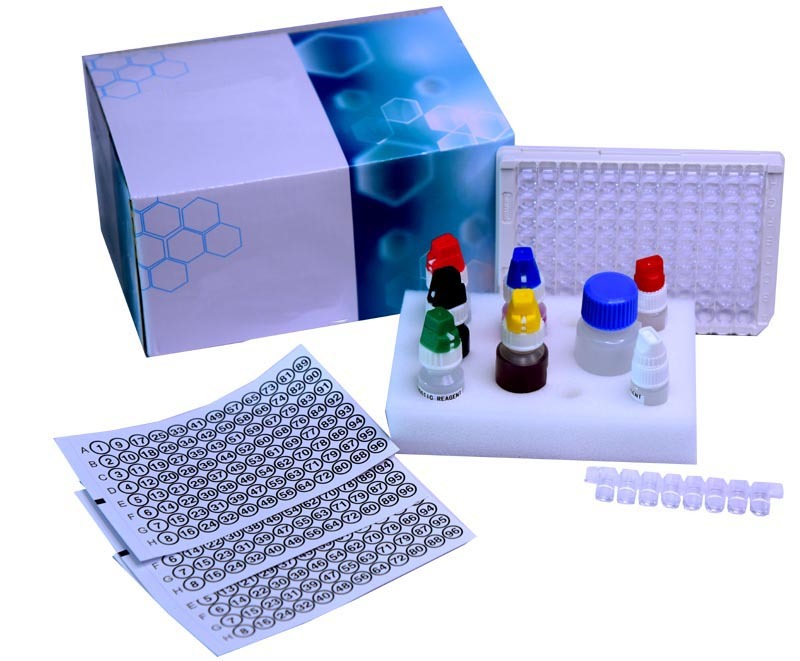
Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit
Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit Specification
- Accuracy
- High sensitivity and specificity (typically >95%)
- Features
- Ready-to-use reagents, colorimetric detection, quantitative results, high reproducibility
- Control Type
- Manual, protocol-based
- Shape
- Rectangular 96-well plate
- Temperature Resistance
- Store at 2C8C. Do not freeze.
- Type
- ELISA kit
- Dimension (L*W*H)
- Standard kit size (96 wells)
- Equipment Type
- In-vitro diagnostic ELISA kit
- Equipment Materials
- High-quality polystyrene microplate, reagent-grade plastics
- Power
- Not required (manual consumable kit)
- Material
- Polystyrene plate, sealed reagents, sample diluent
- Application
- Detection of Epstein Barr Virus VCA IgM antibodies in human serum or plasma
- Packaging
- Sealed box with tamper-resistant packaging
- Kit Components
- Microplate, calibrators/controls, sample diluent, enzyme conjugate, TMB substrate, stop solution, wash buffer
- Incubation Time
- Total assay time: approximately 90120 minutes
- Intended Use
- In-vitro qualitative detection for clinical diagnostic laboratories
- Regulatory Status
- For professional and laboratory diagnostic use only
- Detection Method
- Colorimetric (TMB substrate, read at 450 nm)
- Shelf Life
- 12 months from manufacturing date
- Species Reactivity
- Human plasma or serum
- Limit of Detection
- As per kit insert (typically within clinically relevant range)
- Sample Volume Required
- 10100 L per well
- Quality Control
- Includes positive and negative controls
- Reading Instrument Required
- Microplate (ELISA) reader at 450 nm
- Hazard Information
- Some reagents may contain preservatives, handle as per MSDS
- Assay Principle
- Indirect ELISA for the qualitative determination of VCA IgM antibodies
- Storage Conditions
- 2C8C (refrigerated); keep away from direct sunlight
Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Asia
About Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit
The Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit offers an affordable, cost-effective solution for clinical diagnostic laboratories, blending first-class performance with famed reliability. Designed for the qualitative detection of VCA IgM antibodies, this illustrious kit features ready-to-use reagents for a seamless workflow. With personalised assay versatility, it processes human plasma or serum samples in a standard 96-well microplate, requiring just 10-100 L per sample. Ensuring high sensitivity and specificity, the kit boasts a shelf life of 12 months, tamper-resistant packaging, and effortless colorimetric detection. Manufactured in India by a reputable exporter and supplier, this kit brings premium quality to your lab.
Commercial Utility & Application Features
The EBV VCA IgM ELISA kit provides laboratories with an efficient way to detect Epstein Barr Virus antibodies via a robust indirect ELISA protocol. Used for clinical diagnostics, it suits both high-throughput and focused applications. Key features include easy-to-use colorimetric detection using TMB, a user-friendly manual protocol, and quantitative, reproducible results. Its ready-to-use reagents, quality control (positive and negative controls), and tamper-resistant packaging make it ideal for commercial pathology labs and hospitals.
Delivery Time, Exchange Policy & FOB Information
The EBV VCA IgM ELISA kit is meticulously packed for secure shipment and delivered promptly upon order arrival. Samples provided for evaluation are available under an exchange policy-contact for details. Shipped from the designated FOB Port in India, the kit reaches clients in standard timeframes. Our exchange policy ensures peace of mind on arrival, and all shipments are traceable to guarantee a reliable and timely delivery experience.
Commercial Utility & Application Features
The EBV VCA IgM ELISA kit provides laboratories with an efficient way to detect Epstein Barr Virus antibodies via a robust indirect ELISA protocol. Used for clinical diagnostics, it suits both high-throughput and focused applications. Key features include easy-to-use colorimetric detection using TMB, a user-friendly manual protocol, and quantitative, reproducible results. Its ready-to-use reagents, quality control (positive and negative controls), and tamper-resistant packaging make it ideal for commercial pathology labs and hospitals.
Delivery Time, Exchange Policy & FOB Information
The EBV VCA IgM ELISA kit is meticulously packed for secure shipment and delivered promptly upon order arrival. Samples provided for evaluation are available under an exchange policy-contact for details. Shipped from the designated FOB Port in India, the kit reaches clients in standard timeframes. Our exchange policy ensures peace of mind on arrival, and all shipments are traceable to guarantee a reliable and timely delivery experience.
FAQ's of Epstein Barr Virus VCA IgM (EBV VCA IgM) ELISA kit:
Q: How is the EBV VCA IgM ELISA kit used in diagnostic laboratories?
A: The kit is utilised for the qualitative detection of Epstein Barr Virus VCA IgM antibodies in human plasma or serum, following a manual indirect ELISA protocol.Q: What does the assay process involve in this ELISA kit?
A: The process requires adding 10-100 L of sample per well, incubating with reagents, and using a microplate reader to detect colorimetric changes at 450 nm over 90-120 minutes.Q: When should the EBV VCA IgM ELISA kit not be used?
A: Do not use the kit beyond its 12-month shelf life or if it has not been stored at 2C-8C, as improper conditions may affect its accuracy and reliability.Q: Where is this ELISA kit shipped from and who supplies it?
A: This product is manufactured and exported by a reputable supplier in India, shipped directly from their designated FOB Port to clients worldwide.Q: What are the benefits of using this kit for Epstein Barr Virus detection?
A: Benefits include first-class sensitivity and specificity (over 95%), cost-effectiveness, high reproducibility, ready-to-use reagents, and secure tamper-resistant packaging.Q: How can the sample policy and exchange process be accessed for this kit?
A: Sample policies and exchange options are available on request; details can be obtained by contacting the supplier for terms and eligibility.

Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in ELISA Kits Category
HBcAb IgM ELISA kit
Price 13500 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Box shaped kit
Material : Highgrade laboratory plastics
Anti CCP ELISA Kit
Price 24000 INR / Kit
Minimum Order Quantity : 1 Kit
Shape : Rectangular microplate
Material : Reagents, Enzyme conjugate, Standard controls, Polystyrene plate
Mumps IgG ELISA kit
Price 21450.00 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Rectangular
Material : Plastic
Warranty : yes
Size : Standard
Digoxin ELISA kit
Price 73500.00 INR / Kit
Minimum Order Quantity : 1 Kit
Shape : Rectangular
Material : Plastic
Warranty : yes
Size : Standard





 Send Inquiry
Send Inquiry Send SMS
Send SMS
