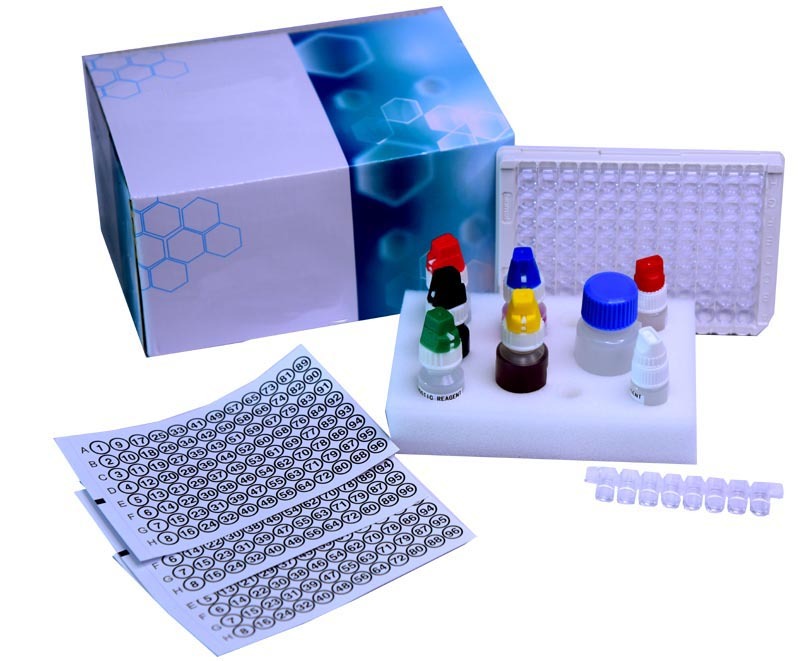
Mumps IgM ELISA kit
Mumps IgM ELISA kit Specification
- Control Type
- Manual by user, following instruction protocol
- Features
- Sensitive, Specific, Reliable, Ready-to-use reagents, Easy-to-follow protocol
- Temperature Resistance
- Store between 2C to 8C
- Accuracy
- High sensitivity and specificity as per kit insert
- Shape
- Rectangular packaging box
- Type
- Immunoassay Kit
- Dimension (L*W*H)
- Standard kit size (box): Approx. 23 cm x 16 cm x 6 cm
- Equipment Type
- ELISA Diagnostic Kit
- Equipment Materials
- Plastic microtiter plate, labeled reagents, buffer solutions
- Power
- Not applicable (manual, non-powered kit)
- Material
- Kit includes plastic, bottles, foil pouches, disposable materials
- Application
- Detection of Mumps virus IgM antibodies in human serum or plasma
Mumps IgM ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
About Mumps IgM ELISA kit
Competitive Advantages and Areas of Use
The Mumps IgM ELISA kit stands out for its high accuracy, sensitivity, and specificity, positioning it as the champion among immunoassay kits for Mumps diagnosis. Used as a qualitative diagnostic test, it is invaluable to clinical laboratories, hospitals, and research institutions. Healthcare professionals and laboratory technicians frequently utilise this special kit to deliver timely, reliable results for patient management and public health monitoring. Its ready-to-use components and easy protocol support seamless workflow integration.
Payment Terms, Export Destinations, and Certifications
The Mumps IgM ELISA kit can be purchased via secure exchange methods, with flexible payment options tailored to client needs. Efficient transport services ensure your kit is safely dispatched to main export markets, including Asia, Africa, Europe, and the Middle East. Each kit is meticulously certified, bearing a CE mark that guarantees compliance with international standards and IVD regulations. Manufacturers provide prompt dispatch and support, ensuring a superior customer experience throughout the procurement process.
FAQ's of Mumps IgM ELISA kit:
Q: How is the Mumps IgM ELISA kit used for detecting Mumps antibodies?
A: The kit employs a sandwich ELISA principle that qualitatively detects Mumps IgM antibodies in patient serum or plasma samples. Users must follow the included manual for sample preparation, reagent addition, incubation, washing, and interpretation of results.Q: What are the primary benefits of utilising this ELISA kit in clinical diagnostics?
A: The main benefits include high sensitivity and specificity for accurate diagnosis, rapid assay completion in approximately 90 minutes, and reliable interpretation, supporting both patient care and outbreak monitoring in various healthcare settings.Q: When should this ELISA kit be chosen for Mumps diagnosis?
A: This kit is ideal when rapid, sensitive, and specific detection of Mumps IgM is required, particularly in suspected cases or during outbreaks where timely intervention and public health response are essential.Q: Where can the Mumps IgM ELISA kit be stored and how is it preserved?
A: For optimal performance, the kit should be stored between 2C and 8C and kept away from light. Unopened kits remain stable for 12 months from the manufacturing date if properly stored as per instructions.Q: What is included in the kit package and what types of samples can be processed?
A: The kit contains a microtiter plate, positive and negative controls, conjugate, substrate, stop solution, sample diluent, wash buffer, and a manual. It is designed to process human serum or plasma samples effectively.Q: How does the manual control type benefit laboratory workflow?
A: Manual control allows laboratory personnel to oversee each step, ensuring precise adherence to protocol, reducing errors, and maintaining test integrity for superior diagnostic outcome.


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
Cytomegalovirus IgG (CMV IgG) ELISA Kit
Price 7800 INR / Kit
Minimum Order Quantity : 1 , , Kit
Shape : Rectangular kit box
Type : Other, ELISA Kit
Material : Plastic, reagent vials, coated microtiter plate
Application : Qualitative and quantitative detection of CMV IgG antibodies in human serum or plasma
Chikungunya IgM ELISA Kit
Price 27150 INR / Kit
Minimum Order Quantity : 10 Kits
Shape : Rectangular kit box
Type : Other, Chikungunya IgM ELISA Kit
Material : Plastic (microplate), glass (reagent bottles), aluminum pouch, paper inserts
Application : Detection of Chikungunya Virus specific IgM antibodies in human serum/plasma
Cortisol ELISA Kit
Price 14400 INR / Kit
Minimum Order Quantity : 1 , , Kit
Type : Other, ELISA Kit
Material : High quality reagents, plastic microplates, enzyme conjugate, and substrate
Application : Quantitative measurement of cortisol levels in biological samples
Androstenedione ELISA kit
Price 25500 INR / Kit
Minimum Order Quantity : 10 Kits
Shape : Rectangular (Microplate)
Type : Other, ELISA Kit
Material : Plastic (Kit Components), Reagents, Microplate
Application : Laboratory Research, Clinical Diagnostics for Androstenedione Quantification in Serum, Plasma, and Other Biological Samples




 Send Inquiry
Send Inquiry Send SMS
Send SMS
