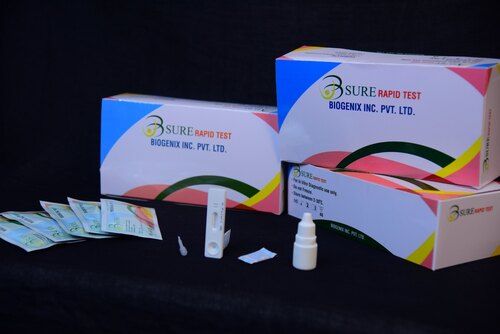
HCV Rapid Test
HCV Rapid Test Trade Information
- Minimum Order Quantity
- 100 Kits
- Supply Ability
- 100000 Kits Per Month
- Delivery Time
- 7 Days
About HCV Rapid Test
BIPL HCV Ab Rapid Test is a double antigen lateral flow chromatographic immunoassay for the qualitative detection of anti-hepatitis C virus antibodies (IgG, IgM, IgA) in human serum, plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HCV.
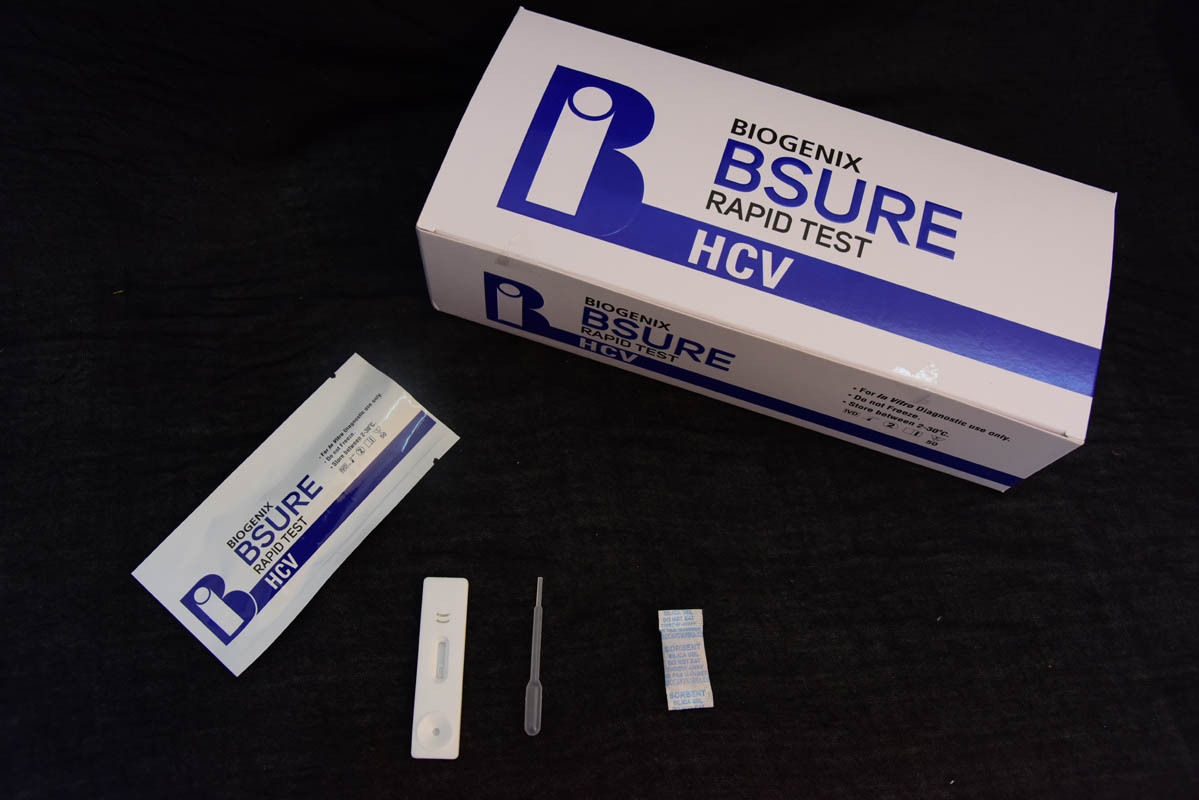
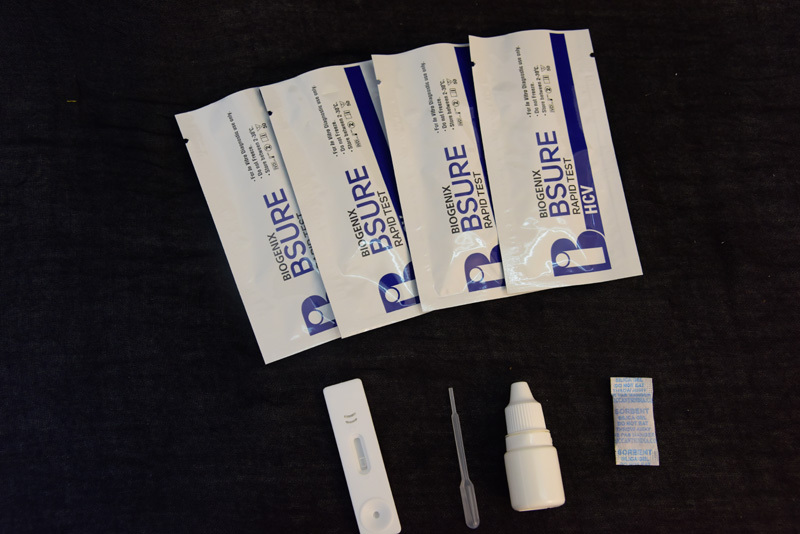
BIPL HCV Ab Rapid Test is a double antigen lateral flow chromatographic immunoassay. The test cassette consists of:
1) a burgundy colored conjugate pad containing recombinant HCV fusion antigen (core, NS3,NS4 and NS5) conjugated with colloidal gold (HCV Ag conjugates) and a control antibody conjugated with colloidal gold,
2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre coated with recombinant HCV fusion antigen (core, NS3, NS4 and NS5), and C line is pre coated with a control line antibody.
Exceptional Diagnostic Confidence
The HCV Rapid Test offers professional-grade reliability, detecting anti-HCV antibodies with remarkable accuracy and speed. Its internal procedural control ensures that users can trust each test result, enabling healthcare professionals to make timely decisions for patient care.
User-Friendly Procedure
Packaged individually for optimal hygiene, the HCV Rapid Test requires minimal sample volume and provides results visually within minutes. The cassette format is straightforward to use, making it an efficient solution for busy clinical environments.
Regulatory Compliance & Safety
Compliant with IVD Directive 98/79/EC and designed for professional in vitro diagnostic use, this test prioritizes safety. Disposal protocols mandate biohazard waste management, ensuring responsible handling throughout its use cycle.
FAQs of HCV Rapid Test:
Q: How is the HCV Rapid Test performed using a serum, plasma, or whole blood sample?
A: The test is carried out by applying 10-100 L of the serum, plasma, or whole blood specimen into the cassette, then adding buffer as per instructions. Results are read visually within 15-20 minutes.Q: What does the internal procedural control line indicate on the test cassette?
A: The internal procedural control line confirms that the test has run correctly and the cassette is functioning as intended, ensuring reliability of results.Q: When should the HCV Rapid Test be used, and who should administer it?
A: The test is designed for professional in vitro diagnostic use only and should be administered by trained healthcare professionals in appropriate clinical settings.Q: Where should used test cassettes and sample materials be disposed of?
A: All used test components and biological samples must be discarded as biohazardous waste in compliance with local safety and regulatory guidelines.Q: What are the primary benefits of choosing the HCV Rapid Test for Hepatitis C antibody detection?
A: Benefits include high sensitivity and specificity (>99%), fast results, minimal sample volume requirements, individual pouch packaging, and easy-to-interpret visual outcome, making it ideal for accurate and efficient testing.Q: How should the HCV Rapid Test be stored to ensure maximum shelf life?
A: Store the test between 2-30C in a dry place to maintain its efficacy throughout its 18-24 month shelf life.Q: Is the HCV Rapid Test compliant with international diagnostic regulations?
A: Yes, the test complies with the IVD Directive 98/79/EC, ensuring it meets stringent international standards for quality and safety.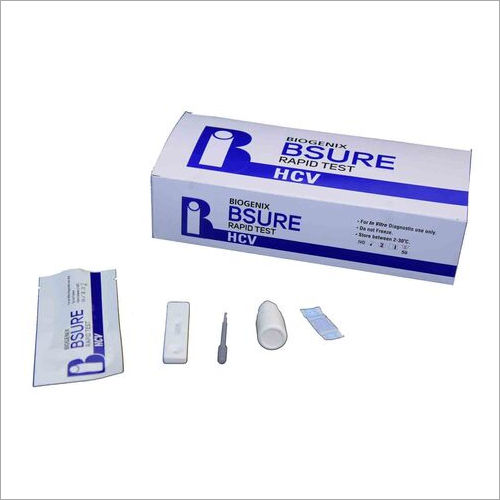

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+


 Send Inquiry
Send Inquiry Send SMS
Send SMS
