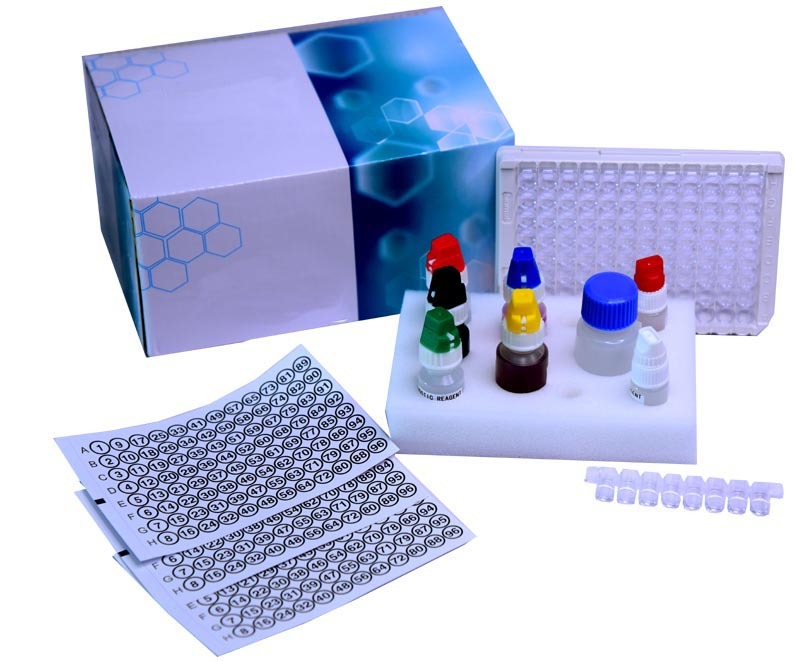
Scrub Typhus IgM ELISA kit
Scrub Typhus IgM ELISA kit Specification
- Shape
- Kit box
- Display Type
- Readout at 450 nm (Microplate Reader, not included)
- Control Type
- Positive and Negative controls included
- Features
- Ready to use, high accuracy, rapid results, user-friendly protocol
- Temperature Resistance
- Reagents stable at 2-8C
- Accuracy
- High Sensitivity and Specificity (>98%)
- Type
- Diagnostic Kit
- Dimension (L*W*H)
- 225 x 150 x 65 mm (approx. kit box size)
- Equipment Type
- ELISA Test Kit
- Equipment Materials
- Kit box with reagents, microtiter plate strips, wash buffer, conjugate, substrate, stop solution, controls
- Power
- Not required (manual processing)
- Material
- Plastic (microtiter strips), reagents in sealed vials
- Application
- Qualitative detection of IgM antibodies to Orientia tsutsugamushi
Scrub Typhus IgM ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 6 Days
- Main Export Market(s)
- Australia, Asia, Central America, North America, Eastern Europe, South America, Africa, Middle East
About Scrub Typhus IgM ELISA kit
Usage, Applications, and Features
The Scrub Typhus IgM ELISA Kit is designed for qualitative detection of IgM antibodies, supporting screening and confirmation of Scrub Typhus infection in hospitals, laboratories, and research centers. With its rapid assay time of approximately 90 minutes, the kit features a user-friendly protocol and ready-to-use reagents. High sensitivity and specificity plus the inclusion of positive and negative controls ensure accuracy and reliability-making it a trusted choice among diagnostic professionals across various application areas.
Packing, Delivery, and Sample Availability
Packing & dispatch of the Scrub Typhus IgM ELISA kit is handled with utmost care, using sturdy kit boxes and cold chain maintenance to preserve reagent quality during goods transport. Customers may request a sample, subject to applicable charges. Each kit is securely packaged for transit, ensuring safe arrival at your location. Delivery times depend on order volume and destination, but prompt shipment is always prioritized to meet urgent diagnostic needs efficiently.
FAQ's of Scrub Typhus IgM ELISA kit:
Q: How is the Scrub Typhus IgM ELISA kit used for detection?
A: The kit employs enzyme-linked immunosorbent assay (ELISA) for qualitative detection of IgM antibodies to Orientia tsutsugamushi in human serum or plasma samples. Results are interpreted via optical density measured at 450 nm.Q: What equipment is required to process the test?
A: No powered equipment is needed for manual processing, but a microplate reader is necessary to measure optical density (OD) at 450 nm for result interpretation.Q: When should the kit components be stored and transported?
A: All reagents should be stored and transported between 2-8C. Cold chain transport is strongly recommended to maintain reagent efficacy and integrity.Q: Where can the kit be effectively applied?
A: This diagnostic kit is suitable for use in hospitals, clinical laboratories, research institutions, and public health centers for both screening and confirmation of Scrub Typhus infection.Q: What are the benefits of using the featured kit controls?
A: Inclusion of positive, negative, and cut-off controls ensures accurate, reliable, and consistent interpretation of results, safeguarding against false positives or negatives.Q: How many samples can be tested with one kit?
A: Each kit contains sufficient materials for up to 96 tests, allowing efficient batch screening and confirmation.


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
Dopa mine Urine Elisa Kit (Fast Track)
Price 66450 INR / Kit
Minimum Order Quantity : 1 Kit
Type : Other, ELISA Kit
Material : Laboratory Grade
Shape : Rectangular kit box
Equipment Type : Diagnostic ELISA Kit
GABA Elisa Kit
Price 124500 INR / Kit
Minimum Order Quantity : 5 Kits
Type : Other, Elisa Kit
Material : Plastic (Microplate), reagents in buffer solutions
Shape : Rectangular (Microplate format)
Equipment Type : Biochemical Assay Kit
Cortisol ELISA Kit
Price 14400 INR / Kit
Minimum Order Quantity : 1 , , Kit
Type : Other, ELISA Kit
Material : High quality reagents, plastic microplates, enzyme conjugate, and substrate
Equipment Type : Diagnostic kit
E.coli Verotoxin (Fecal) ELISA kit
Price 36450.00 INR / Kit
Minimum Order Quantity : 50 Kits
Type : Other, Elisa Kits
Shape : Round




 Send Inquiry
Send Inquiry Send SMS
Send SMS
