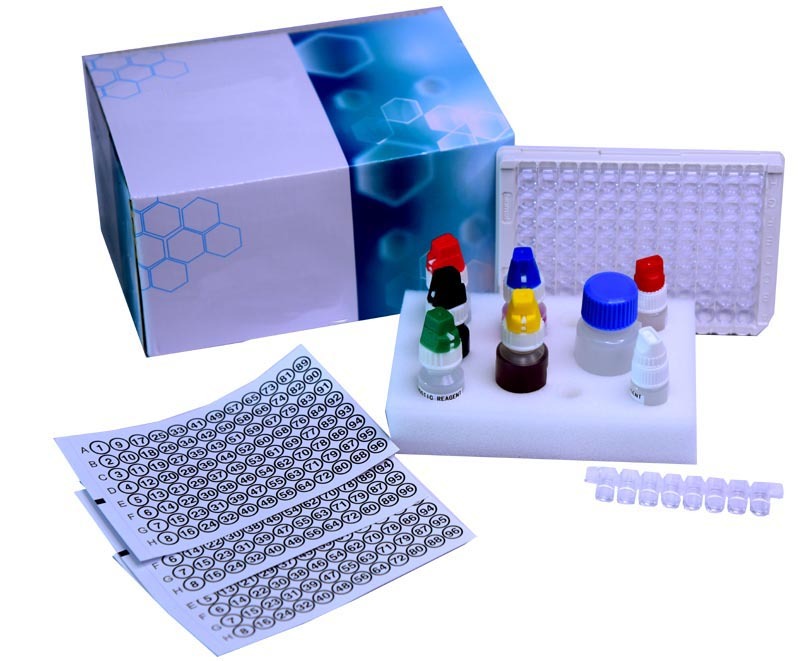
HIV 1/2 ELISA kit
HIV 1/2 ELISA kit Specification
- Accuracy
- >99% sensitivity, >99% specificity
- Temperature Resistance
- Store at 2C 8C
- Shape
- Rectangular packaging
- Control Type
- Manual, using microplate reader
- Features
- Ready to use reagents, High sensitivity and specificity, Easy usage protocol, CE/ISO certified
- Type
- Diagnostic ELISA Kit
- Dimension (L*W*H)
- Box packaging, approx. 20cm x 15cm x 5cm
- Equipment Type
- ELISA kit
- Equipment Materials
- Plastic, Paper, Silica Gel
- Material
- Biological/chemical reagents, microplate strips
- Application
- Detection of HIV 1/2 antibodies in serum/plasma
- Regulatory Approval
- CE, ISO 13485 and WHO prequalified
- Incubation Time
- Total assay time ~2 hours
- Sample Volume Requirement
- 50-100 L per test
- Controls Included
- Positive and negative control included
- Shelf Life
- 18 months from manufacturing date
- Pack Size
- 96 tests per kit
- Storage Condition
- 2C to 8C
- Reading Wavelength
- 450 nm (reference 630 nm)
- Minimum Detection Limit
- Complies with international standards for serological HIV detection
- Detection Method
- Enzyme Linked Immunosorbent Assay (ELISA)
- Accessories Included
- Microplate strips, reagents, controls and instruction manual
- User Level
- Laboratory professional
- Expiry Date Indication
- Stamped on kit label
- Hazard Information
- Non-hazardous under normal laboratory conditions
- Assay Format
- Indirect
- Barcode Availability
- On packaging for tracking
HIV 1/2 ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
About HIV 1/2 ELISA kit
Material Features, Application & Commercial Uses
Constructed with high-grade plastic, paper, silica gel, and biological/chemical reagents, the HIV 1/2 ELISA Kit achieves remarkable performance in detecting HIV 1/2 antibodies in serum or plasma. Its precise application supports clinical laboratories, diagnostic centers, and commercial testing facilities, ensuring dependable results and streamlined workflow. The kit's advanced design and comprehensive accessories facilitate commercial use in routine screening, epidemiological surveys, and quality assurance programs, enhancing healthcare efficacy and safety.
FOB Port, Delivery & Packaging Details
Orders for the HIV 1/2 ELISA Kit can be handed over at major Indian FOB ports. Delivery time is prompt, typically aligning with the proposal amount and expenditure agreed upon in procurement contracts. Each kit comes securely packed in sturdy rectangular boxes (20cm x 15cm x 5cm) with barcode labeling for efficient tracking and expiry date indication. The packaging ensures all accessories and reagents are intact, safeguarding sample integrity and quality throughout transit and delivery.
FAQ's of HIV 1/2 ELISA kit:
Q: How does the HIV 1/2 ELISA kit ensure high accuracy in antibody detection?
A: The kit follows an indirect ELISA format with enzyme-linked detection, achieving more than 99% sensitivity and specificity. It includes both positive and negative controls, ensuring reliable and reproducible results for HIV 1/2 antibody screening.Q: What materials and accessories are included in each kit?
A: Every kit contains microplate strips, ready-to-use reagents, positive and negative controls, silica gel, an instruction manual, and sturdy plastic and paper packaging to maintain product integrity and usability.Q: When can laboratory professionals use the HIV 1/2 ELISA kit after delivery?
A: The kit is ready for use immediately upon receipt, provided it is stored at the recommended temperature of 2C to 8C. All reagents and accessories are pre-packed and labeled for instant setup.Q: Where is the HIV 1/2 ELISA kit typically applied in commercial or clinical settings?
A: This kit is ideal for use in hospital laboratories, blood banks, diagnostic centers, and research institutions where precise serological HIV detection is crucial for patient screening and disease management.Q: What is the process for using the HIV 1/2 ELISA kit?
A: Laboratory professionals add serum/plasma samples (50-100 L), incubate for approximately 2 hours, and read results at a 450 nm wavelength using a microplate reader, following the manual control protocol included.Q: What benefits does the kit offer in terms of standards and compliance?
A: The HIV 1/2 ELISA kit complies with international regulatory standards, including CE, ISO 13485, and WHO prequalification, ensuring product quality, traceability, and reliability in diverse operational environments.


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
Progesterone ELISA Kit
Price 13500 INR / Kit
Minimum Order Quantity : 1 , , Kit
Material : Highgrade plastic, reagents, buffers
Equipment Materials : Kit comprises precoated 96well plate, buffers, reagents, substrate solution, conjugate, standard solution
Type : Other, ELISA Kit
Measles IgG ELISA kit
Price 21450 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Rectangular box (Kit packaging)
Material : Highgrade Microplate, reagents
Equipment Materials : Plastic wells, reagents, microplate
Type : Other, ELISA Kit
Japanese Encephalitis IgM ELISA Kit
Price 43500 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Kit box with flat microplate wells
Material : Microplates, reagent vials, buffer bottles
Equipment Materials : Highquality plastic and coated microplates
Type : Other, IgM ELISA Kit
HSV-2 IgG ELISA kit
Price 7800.00 INR / Kit
Minimum Order Quantity : 50 Kits
Shape : Rectangular
Material : Plastic






 Send Inquiry
Send Inquiry Send SMS
Send SMS
