Measles IgM ELISA kit
Measles IgM ELISA kit Specification
- Shape
- Rectangular strips in a compact box
- Accuracy
- Qualitative detection: Highly sensitive and specific
- Temperature Resistance
- Store at 2C to 8C
- Control Type
- Manual
- Features
- Ready-to-use reagents; 96-well format; high sensitivity and specificity; colorimetric detection
- Type
- Measles IgM ELISA Kit
- Dimension (L*W*H)
- Box size: Approx. 20 cm x 15 cm x 8 cm
- Equipment Type
- Diagnostic ELISA Kit
- Equipment Materials
- High-quality plastic, microtiter well strips
- Power
- Not required (manual assay)
- Material
- Plastic (wells), Paper (manual), Reagents (liquid form)
- Application
- Measles IgM detection in human serum/plasma
- Incubation Time
- Approximately 6090 minutes total
- Number of Tests
- Up to 96 samples per kit
- Shelf Life
- 1218 Months (unopened at 28C)
- Intended Use
- For in vitro diagnostic use only
- Minimum Sample Volume
- 100 L
- Sample Type
- Serum / Plasma
- Test Principle
- Enzyme-Linked Immunosorbent Assay (ELISA) for qualitative detection of anti-measles IgM
- Kit Components
- Microtiter plate, positive / negative / cut-off controls, conjugate, substrate, wash buffer, stop solution, instruction manual
- Detection Method
- Colorimetric, using enzyme-substrate reaction
- Reading Method
- Microplate reader at 450 nm (reference 620650 nm)
Measles IgM ELISA kit Trade Information
- Minimum Order Quantity
- 50 Kits
- Payment Terms
- Cash Advance (CA), Cash in Advance (CID)
- Supply Ability
- 50000 Kits Per Week
- Delivery Time
- 7 Days
- Main Export Market(s)
- Asia, Australia, Central America, South America, Eastern Europe, North America, Western Europe, Middle East, Africa
About Measles IgM ELISA kit
Versatile Usage and Application Surface
The Measles IgM ELISA kit is trusted by diagnostic laboratories, hospitals, and clinical research centers. Its application surface encompasses human serum and plasma samples, making it ideal for a wide range of in vitro diagnostic investigations. Used by trained laboratory technicians, this compact, ready-to-use kit supports accurate detection on flat microtiter wells, ensuring consistent results and streamlined workflow in diverse clinical environments.
Export, Delivery, and Domestic Supply
The Measles IgM ELISA kit boasts robust export capabilities, efficiently serving international clients in Europe, Asia, Africa, and other markets. With a strong supply ability, we ensure seamless goods transport, precise logistics handling, and prompt quotation response. Our streamlined distribution network guarantees timely delivery to domestic markets across India, catering to hospitals and laboratories seeking reliable diagnostic solutions.
FAQs of Measles IgM ELISA kit:
Q: How does the Measles IgM ELISA kit detect anti-measles IgM antibodies?
A: The kit uses an enzyme-linked immunosorbent assay (ELISA) method with colorimetric detection, allowing qualitative identification of anti-measles IgM in human serum or plasma through an enzyme-substrate reaction.Q: What types of samples can be used with this ELISA kit?
A: This kit is suitable for use with serum or plasma samples collected from patients, requiring a minimum sample volume of 100 L per test.Q: When should I use the Measles IgM ELISA kit in a clinical setting?
A: The kit is intended for use when there is a need to confirm or rule out measles infection by detecting patient-specific IgM antibodies during clinical investigations or outbreak situations.Q: Where is the ideal site of application for the Measles IgM ELISA kit?
A: The kit is designed for in vitro diagnostic use and is ideally applied in hospital laboratories, diagnostic centers, and research institutions equipped with a microplate reader.Q: What are the benefits of using this kit compared to other methods?
A: This kit offers high sensitivity and specificity, processes up to 96 samples rapidly, and provides reliable results using ready-to-use reagents, making it efficient and cost-effective for large-scale diagnostic testing.Q: How long does the entire assay process take from start to finish?
A: The total incubation and assay time typically ranges from 60 to 90 minutes, enabling quick turnaround of diagnostic results.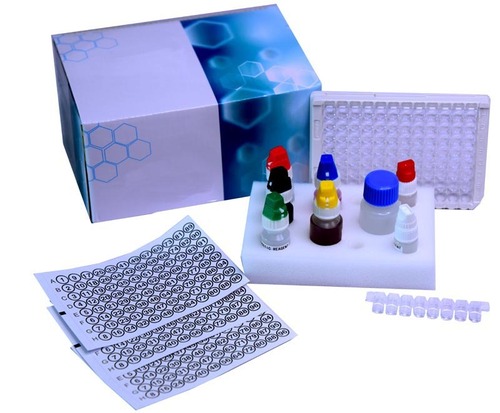



Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in ELISA Kits Category
Dopa mine Urine Elisa Kit (Fast Track)
Price 66450 INR / Kit
Minimum Order Quantity : 1 Kit
Material : Laboratory Grade
Shape : Rectangular kit box
Features : Fast Track protocol, readytouse reagents, high throughput testing
Temperature Resistance : Storage: 28C
Toxocara IgG ELISA kit
Price 67500 INR / Kit
Minimum Order Quantity : 50 Kits
Material : Microplate, reagents, buffer solutions
Shape : Kit box, rectangular
Features : Readytouse components, controls included, microplate format, breakable wells, high sensitivity, clear instructions
Temperature Resistance : Store at 28C (do not freeze)
West Nile IgM ELISA kit
Price 54450 INR / Kit
Minimum Order Quantity : 50 Kits
Material : Biological reagents, microtiter plate, buffers, conjugate, substrate
Shape : Rectangular kit box
Features : Readytouse reagents, colorimetric detection, includes positive and negative controls, easytofollow protocol
Temperature Resistance : Store at 28C, do not freeze
17-OH Progesterone ELISA kit
Price 18450 INR / Kit
Minimum Order Quantity : 10 Kits
Material : Plastic microplates and reagent vials
Shape : Rectangular microplate
Features : Readytouse reagents, high sensitivity, suitable for clinical diagnostics
Temperature Resistance : Store at 28C





 Send Inquiry
Send Inquiry Send SMS
Send SMS
